Food and Nutrition Sciences
Vol.4 No.8(2013), Article ID:35452,9 pages DOI:10.4236/fns.2013.48111
Effect of Pure Culture Fermentation on Biochemical Composition of Moringa oleifera Lam Leaves Powders
![]()
1Nationnal School of Agro-Industrial Sciences, Ngaoundéré, Cameroon; 2University Institute of Technology, Ngaoundéré, Cameroon; 3UMR 95 Qualisud, CIRAD, Montpellier, France.
Email: *tatsadjeu@yahoo.fr
Copyright © 2013 Noumo Ngangmou Thierry et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 20th, 2013; revised June 20th, 2013; accepted June 27th, 2013
Keywords: Moringa Oleifera; Leaf Powder; Fermentation; Lactobacillus Plantarum A6; Availability; Protein Digestibility
ABSTRACT
This study was carried out to determine the effect of the age of the leaves and fermentation on in vitro protein digestibility and biochemical properties of leaves powder of Moringa oleifera. A 6 × 2 × 2 factorial design with two ages of the leaves (one and seven-month-old leaves), six times of fermentation and two fermentation temperatures was used for this purpose. One and seven-month-old fresh leaves were dried at 45˚C for 24 h, crushed to 1000 µm then fermented at 30˚C and 37˚C for 120 hours with Lactobacillus plantarum A6 at 108 CFU/g. Samples were withdrawn every 24 hours for physico-chemical analyses. Results showed that 7 month-old leaves were richer in iron, proteins, polyphenols and phytates than one month old leaves. The phytates content dropped from 66.92% and 61.95% in the seven and one month-old leaves powders respectively fermented at 37˚C, and from 54.15% and 67.95% in the seven and one month-old leaves powders respectively fermented at 30˚C. Protein content increased by 26.34% and 24.48% for the 1- and 7-month-old leaves powders respectively fermented at 37˚C, and by 13.06% and 13.97% for the 1- and 7- month-old leaves powders respectively, fermented at 30˚C. Iron availability increased from 35.97% to 40.57% and 20.74% to 30.98% for the 1- and 7-month-old leaves powders respectively, fermented at 37˚C and from 35.97% to 39.79% and 20.76% to 23.72% for the 1- and 7-month-old leaves powders respectively, fermented at 30˚C. There was a negative correlation between pH, total and reducing sugar contents, time as well as fermentation temperature, whereas there was a positive correlation between total protein content and pepsic digestibility of protein and fermentation time. From these results, fermentation of M. oleifera leaf powder by Lactobacillus plantarum A6 increases protein content, pepsic digestibility of protein and availability of iron and reduces the phytates content of these powders.
1. Introduction
In recent times malnutrition is still one of the major concerns in developing countries [1]. In Africa, in 2012, there were about 200 million under fed with an increase of 20% compared to the year 1990 [2]. The analysis of the causes of malnutrition in Africa showed that the most alarming nutritional problems are those of food deficiencies of proteins and micronutrients [3]. Indeed, the diets of many African populations are mainly made up of products of plant origin and often rest on the transformation and consumption of mainly starch-based species [4]. This lack of food diversity often leads to nutritional deficiencies [5]. The reduction of malnutrition thus requires a design of fast solutions of which one is the research of the local resources of plant proteins [3].
In many countries of Africa and Asia, due to its capacity to resist the dryness and their high amounts of nutriaents, Moringa oleifera leaves represent a significant local plant source of nutrients such as proteins, vitamins and minerals [6]. M. oleifera is used in several areas of the world as food and to fight food deficiency diseases or to enrich various foods in proteins, iron, calcium, manganese and zinc [7]. However, although rich in nutrients, M. oleifera leaves contain anti-nutritional factors (ANF) in particular phytates, tannins, saponins and fibres [8] which reduce the bioavailability of nutrients [9].
The technological processes such as mechanical, thermal, chemical and biological processes are used to reduce ANF content and improve the bioavailability of the nutrients. Among these processes, the biological processes such as fermentation, contrary to thermal, chemical and mechanical processes which can deteriorate quality of food, constitutes an effective means to reduce ANF content of foods [4] and in addition to increase the bioavailability of nutrients, also improves organoleptic and sanitary properties [10] and preservation of food [11].
Among the microorganisms used in food fermentation, lactic bacteria represent the principal group and are found on various substrates [12]. In this group L. plantarum is found in the fermentation of corn pastes [13], sauerkraut, ogi and several other fermented food of vegetable origin [14]. In addition to its abilities to reduce ANF content in food such as phytates due to the production of phytase [15], fibre by the b-galactosidase [16,17], L. plantarum used as ferment increases protein content, bioavailability of nutrients, improves shelf life of food [18-20] and increases the energy density of rich starch foods [21].
As part of main study aimed on improving the nutriational qualities of Moringa oleifera leaves, the present study was carried out to evaluate the effect of fermentation by Lactobacillus plantarum A6 on chemical composition and nutritional properties of Moringa oleifera leaves powder.
2. Materials and Methods
2.1. Raw Material
The raw material (fresh M. oleifera leaves) was collected at Maroua town (Far-North region of Cameroon) in December 2012. Two groups of leaves were collected: The first group constituted by one (01)-month-old leaves considered in this work as “young leaves”. The second group made up with seven (07)-month-old leaves considered as “old leaves”.
2.2. Starter
L. plantarum A6 was provided by the Microbiology Laboratory of CIRAD Montpellier, France.
2.3. Production of Leaves Powders
The fresh leaves of M. oleifera were sorted to eliminate the impurities, fade and dead leaves then washed with tap water. Thereafter, these leaves were washed with distilled water and were drained on plastic trays. The leaves were then dried at 45˚C ± 2˚C for 24 h in a ventilated hot air dryer (CK2000AUF). The dried leaves were crushed in a hammer mill (standard Culatti, Polymix, Germany) through a sieve mesh of 1000 µm.
2.4. Culture of L. plantarum A6
Stock culture of Lactobacillus plantarum A6 was obtained from UMR of CIRAD Montpellier, France. This strain was propagated in MRS Broth (pH 5.5) at 37˚C for 16 h. The culture obtained was centrifuged at 6500 rpm for 20 minutes at 4˚C and the bottom used as starter.
2.5. Fermentation
Approximately 200 g of leaves powder of each age were introduced into glass bottles of 1000 ml, and then sterilized at 121˚C for 20 min. After cooling, the powders were inoculated with L. plantarum A6 at 108 CFU/g. The water content of medium was based on water absorption capacity of powders. The mixtures were homogenized in aseptic condition using a sterile glass rod. Fermentation was carried out at 30˚C and 37˚C for 120 hours. Samples without bacteria were set up at each temperature of fermentation as control.
2.6. Chemicals Analysis
For the analysis, 50 g of samples were withdrawn every 24 h and dried at 45˚C for 24 hours. Dried fermented and non-fermented powders were analyzed for moisture, proteins, ash and lipids content essentially according to standards methods of the Association of Official Analytical Chemists (AOAC) [22,23]. Powders samples were acid-hydrolyzed and the resulting reducing sugar designnated as available carbohydrates, was determined by the dinitrosalicylic acid (DNS) method of Fisher and Stein [24]. Total sugars were extracted and measured by spectrophotometry using Dubois et al. method [25]. Total protein content was determined by Devani method [26] after sulfuric acid mineralization of samples in presence of selenium catalyst, with a coefficient of conversion of nitrogen into protides of 6.25. Total lipids were quantified using method described by Bourely [27] and the total iron content using Rodier method [28]. The phytic acid content was estimated using a modified method of McCance and Widdowson as described by Abiodun et al. [29] while the phenolic compounds were estimated by the method of Marigo [30].
In vitro pepsic digestibility of protein (IVPDP) was determined using modified Mertz et al. [31] method. In this procedure 1 g of sample was mixed with 35 ml of 0.1 M phosphate buffer with pepsin (1.5 g pepsin/L pH 2.0), incubated for 2 hrs in a shaking water bath at 37˚C and centrifuged at 10,000 rpm for 15 min. The residue was washed in 10 ml of phosphate buffer and re-centrifuged at 10,000 rpm for 15 minutes. The supernatants were collected and their proteins content’s determined by the method of Lowry et al. [32]. The IVPDP was determined as a proportion of soluble proteins after pepsic digestion.
Iron availability was determined in 1 mL of supernatant obtained after peptic digestion of one gram of leaf powder according to the method described by Lestienne [4] with some modifications. Practically, 1 mL of the supernatant of peptic digestion was introduced into a test tube in which was added 1 mL of hydrochloric acid (1 N), 0.5 mL of sodium acetate saturated solution, 0.3 mL of ascorbic acid and 1mL of orthophenantrolin (0.1%). The mixture was shaken and heated for 5 min at 100˚C in a water bath. After cooling, the volume was completed to 10 ml and the absorbance read at 540 nm against a blank. The standard curve was carried out using Mohr salt [(NH4)2, Fe (SO4)2, 6H2O].
Water absorption capacity (WAC) of leaves powders was determined according to method of American Association of Cereal Chemist (AACC) 44-1A [33].
2.7. Statistical Analysis
The experiment was carried out in triplicate and data obtained were analyzed by analysis of variance in Statistica software [34]. Differences between means were tested using the Duncan Multiple Range Test and correlations between variables were tested using correlation table of Pearson.
3. Results and Discussion
3.1. Chemical Composition and Water Absorption Capacity of M. oleifera Leaves Powders
The results for proximate composition and the Water absorption capacity of M. oleifera are shown in Table 1. The phosphorus, ash, total polyphenol, total lipid and phytates content of M. oleifera leaves powders analyzed vary (p < 0.05) according to the age of the leaves.
The increase in phosphorus content from 17.18 ± 0.71 g/100g DM to 26.57 ± 1.11 g/100g DM, in total polyphenols from 0.92 ± 0.18 g/100g DM to 3.75 ± 0.84 g/100g DM and in phytates from 699 ± 13 mg/100g DM to 1182.25 ± 21.69 g/100g DM were observed from young to old leaves. The ash, lipids and reducing sugars contents decrease respectively from 13.67 ± 0.33 g/100g DM to 12.68 ± 0.51 g/100g DM; 16.091 ± 0.52 to 9.34 ± 0.67 g/100g DM and from 4.35 ± 0.025 g/100g DM to 3.73 ± 0.32 g/100g DM from young to old leaves. However, dry matter, total sugars and crude fibres contents as well as WAC remain constant in the old and the young leaves powders. Total sugars contents (13.02 ± 0.79 g/100g DM and 13.62 ± 0.54 g/100g DM respectively for young and the old leaves powders) are near to those reported by Ray-Yu et al. [35] (14.47 ± 2.68 g/100g MS and 13.20 ± 1.98 g/100g MS). The phosphorus contents are lower than the value reported by Pallavi and Dipika [36] (203 mg/100g) obtained in sun dried leaves of

Table 1. Chemical composition and water absorption capacity of powders of M. oleifera leaves.
M. oleifera. On the other hand, the DM content is greater than the value reported by Moyo et al. [37] (90.47%) on M. oleifera leaves powder obtained after air-drying and milling green leaves.
The crude fibres content varied from 11.63% to 13.02% respectively for young and old leaves powders. These values are close to those reported by Tchiégang and Aïssatou [38] (12.03%), but are lower than that found by Price [39] (19.2%) and Broin [40] (15%) in M. oleifera leaves.
The lipid content of the old-leaves powders (9.34 ± 0.67 g/100 g DM) is near to the value reported by Richter et al. [41] (10.6 g/100g DM) on the leaves powder of M. oleifera of Niger, but higher compared to lipid content reported by Tchiégang and Aïssatou [38] (5.17 ± 0.01g/ 100g DM) in M. oleifera leaves collected in the locality of Bini-Dang (Adamawa-Cameroun).
The ANF (total polyphenols and phytates) were more concentrated in the old-leaves powders as shown by Ray-Yu et al. [35]. The young leaves powder has 0.92 ± 0.18 g/100g DM of total polyphenol however the oldleaves powder the polyphenol content is 3.75 ± 0.84 g/ 100g DM similar to the value reported by Ray-Yu et al. [35] (3.06 ± 0.01 g/100g DM). The phytates contents varied from 699 mg/100g DM in young leaves powder to 1182.25 mg/100g DM in old leaves powder; values lower than that found by Foidl [42] (3.1 g/100g DM) in those leaves. Amaglo et al. [43] studied the effect of age on chemical composition of M. oleifera leaves and showed an increase in protein and phytochemical compounds content in the leaves with the age of the leaves due to their accumulation during the growth of plants. However, the differences in nutrients and anti-nutrients contents obtained with others authors would be due to the agroecological differences of the analyzed species, the seasons of harvests or the ages of these leaves.
3.2. Effect of Fermentation on Physico-Chemical and Nutritional Properties of M. oleifera Leaves Powders
3.2.1. Changes in pH
The changes in the pH during fermentation of M. oleifera leaves powders are presented in Figure 1. The fermentation conditions have a significant effect (p < 0.05) on the pH. Fermentation Time, age of leaves, temperature and their interactions influence significantly the pH. Generally, the pH changes in two phases. In the first phase a slight reduction in the pH during the first 72 hours of fermentation. This fall goes from 5.8 to 5.4 for old leaves powders fermented at 37˚C and from 5.8 to 5.2 for young leaves powders fermented at 30˚C. In a second phase, there is an increase in the pH from the 72nd hour of fermentation. This increase reaches values of 6.4 for the old leaves powder fermented at 37˚C and 7.1 for the young leaves powders fermented at 30˚C. The reduction (p < 0.05) in the pH of the powders during the first 72 hours of fermentation, would be due to a production of organic acids in the medium especially lactic acid resulting from the metabolic activity of L. plantarum A6 [44]. After 72 hours of fermentation, there is a rise in the pH from the 72nd hour until the end of fermentation (120 Hours). The increase of the pH observed in this second phase would be due either to the increase of proteins content of the

Figure 1. Changes in pH during fermentation (O37 = powders of 7-month-old leaves (old) fermented with 37˚C; O30 = powders of 7-month-old leaves (old) fermented with 30˚C, Y37 = powders of leaves of 1 month (young leaves) fermented with 37˚C; Y30 = powders of leaves of 1 month (young leaves) fermented with 30˚C).
leaves powders or to the proteolytic activities of the lactic bacteria [45], releasing peptides, amino acids and ammonia [46] which increases the pH of the medium.
3.2.2. Total Sugars
The changes in total sugars during fermentation are shown in Figure 2.
There was a drop of total sugars contents from 14.12 g/100g DM to 9.39 g/100g DM and from 14.12 g/100g DM to 10.41 g/100g DM for the young leaves powders respectively fermented at 37˚C and 30˚C. For the old leaves powders, total sugar content varied from 12.73 g/100g DM to 8.52 g/100g DM and from 12.73 g/100g DM to 9.67 g/100g DM when they were fermented respectively at 37˚C and 30˚C.
The drop of the total sugars contents would be due to their metabolism by L. plantarum A6 during fermentation. Indeed the lactic bacteria use sugars as energy sources during fermentation with production of organic acids [47-49]. Moreover L. plantarum have pectinases [49,50] and α-amylase. Thus, there are able to metabolized complex sugars.
3.2.3. Total Polyphenols
The changes in total polyphenols during fermentation are shown in Figure 3. There is no significant variation of polyphenols contents during fermentation (p > 0.05). However, several authors noticed decrease of polyphenol content during fermentation.
Indeed, Medoua [51] obtained 48.4% of total poly-

Figure 2. Changes in total sugars during fermentation (O37 = powders of 7-month-old leaves (old) fermented with 37˚C; O30 = powders of 7-month-old leaves (old) fermented with 30˚C, Y37 = powders of leaves of 1 month (young leaves) fermented with 37˚C; Y30 = powders of leaves of 1 month (young leaves) fermented with 30˚C).

Figure 3. Changes in total polyphenol during fermentation (O37 = powders of 7-month-old leaves (old) fermented with 37˚C; O30 = powders of 7-month-old leaves (old) fermented with 30˚C, Y37 = powders of leaves of 1 month (young leaves) fermented with 37˚C; Y30 = powders of leaves of 1 month (young leaves) fermented with 30˚C).
phenol losses after 14 days of fermentation of the hardened tubers of yams (Dioscorea dumetorum). These differences may result in absence of enzymes responsible of the phenolic compounds hydrolysis during fermentation such as the polyphenols oxydases, peroxidases, laccases. The reduction of the polyphenols contents during the fermentation of food is due to the presence of these enzymes which may be present in food stuff or produce by the micro-organisms during fermentation [4].
3.2.4. Total Protein
The evolution of total proteins during fermentation is represented by Figure 4. There is a general increase in total protein content (p < 0.05). However, this increase varies with the age of the leaves and the temperature of fermentation. Increases from 38 g/100g DM to 44 g/100g DM for the old leaves powders fermented at 37˚C and from 33 g/100g DM to 39 g/100g DM for the young leaves powders fermented at 37˚C were noticed.
The improvement of the protein value of food during fermentation has been reported by several authors [52- 55].
The increase in the protein content would be due either to the increase in the biomass supported in this case by the pH of the powders during the fermentation which is in the interval of optimal pH of the lactic bacteria [13], or the reduction of the amount of dry matter [56] with consumption of component such as sugars.
3.2.5. Changes in Phytates
The changes in phytates contents of the different powders during fermentation are shown in Figure 5. The different factors of leaves ages, temperatures of fermentation and fermentation time affect phytates contents (p < 0.05) of powders during fermentation. There is a negative correlation (p < 0.05) between the phytates contents, the temperature of fermentation (r = −0.53) and the fer-
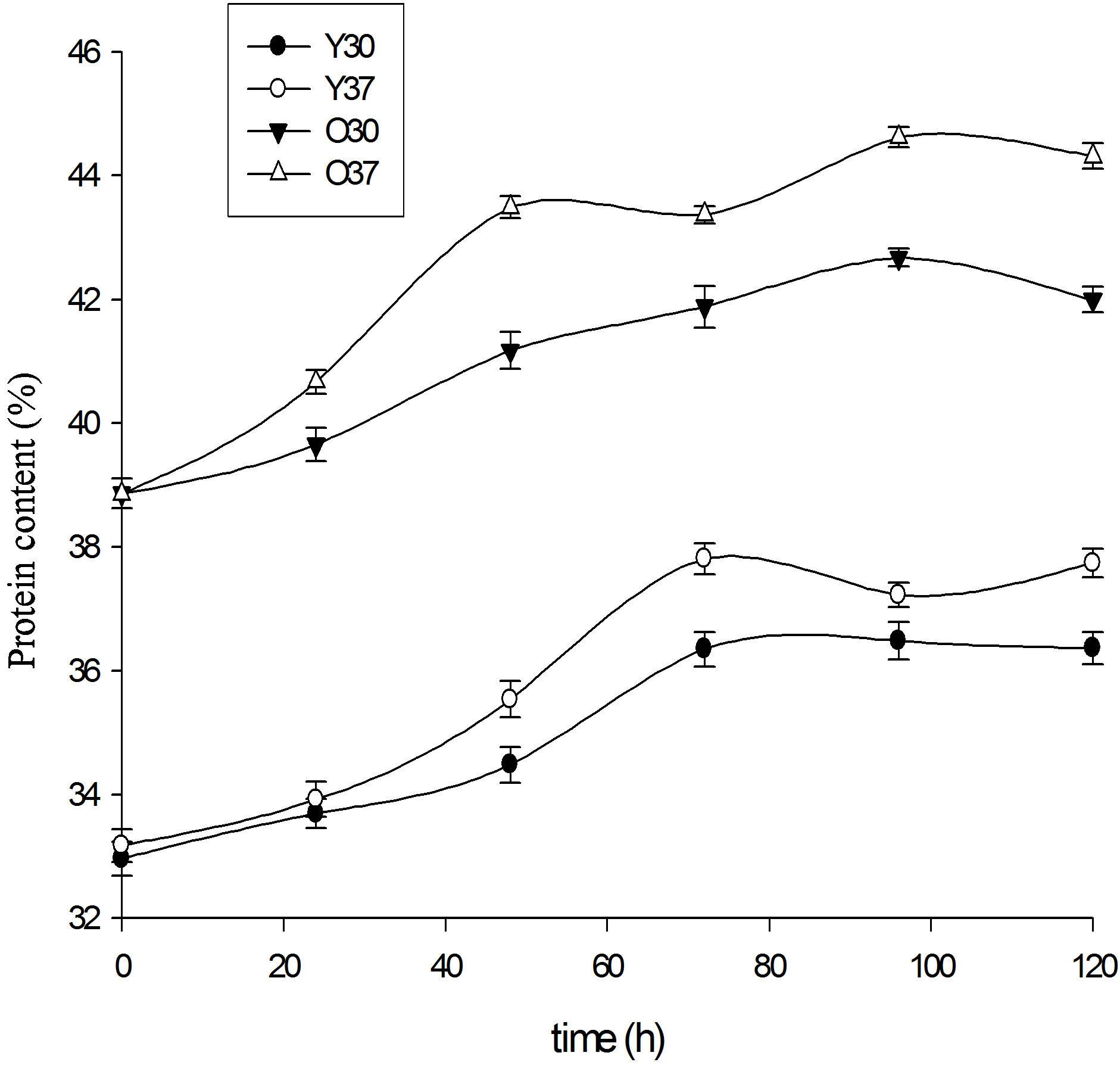
Figure 4. Changes in total protein during fermentation (O37 = powders of 7-month-old leaves (old) fermented with 37˚C; O30 = powders of 7-month-old leaves (old) fermented with 30˚C, Y37 = powders of leaves of 1 month (young leaves) fermented with 37˚C; Y30 = powders of leaves of 1 month (young leaves) fermented with 30˚C).

Figure 5. Evolution of phytates contents during fermentation (O37 = powders of 7-month-old leaves (old) fermented with 37˚C; O30 = powders of 7-month-old leaves (old) fermented with 30˚C, Y37 = powders of leaves of 1 month (young leaves) fermented with 37˚C; Y30 = powders of leaves of 1 month (young leaves) fermented with 30˚C).
mentation time (r = −0.72). A reduction of 66.92% (from 1182 mg/100g DM to 391 mg/100g DM) of phytates contents in old-leaves powders fermented at 37˚C was noticed whereas a drop was from 1182 mg/100g DM to 542 mg/100g DM when the old-leaves powders were fermented at 30˚C. In the young leaves powders, the decrease from 699 mg/100g DM to 266 mg/100g DM (61.95%) and from 699.42 mg/100g DM to 224.76 mg/ 100g DM were noticed with fermentation at 37˚C and 30˚C respectively. Several authors also noted a reduction of phytates contents in fermented food [57-59]. The reduction of phytates contents during fermentation could be allotted to the production of phytases during fermentation by L. plantarum [60].
3.2.6. In Vitro Pepsic Digestibility of Proteins of M. oleifera Fermented Leaves Powders
The Figure 6 shows the in vitro peptic digestibility proteins (IVPDP) of M. oleifera leaves powders during fermentation. There is a general increase of IVPDP of M. oleifera leaves powders during fermentation. The factors age of leaves, temperature and time of fermentation have a significant effect (p < 0.05) on IVPDP. The increases of IVPDP from 34.72% to 55.46% and 53.08% were observed for the young leaves powders respectively fermented at 37˚C and 30˚C. In the old-leaves powders, the increases were from 39.97% to 63.97% and 55.57% when they were fermented respectively at 37˚C and 30˚C.

Figure 6. Protein pepsic digestibility during fermentation (O37 = powders of 7-month-old leaves (old) fermented with 37˚C; O30 = powders of 7-month-old leaves (old) fermented with 30˚C, Y37 = powders of leaves of 1 month (young leaves) fermented with 37˚C; Y30 = powders of leaves of 1 month (young leaves) fermented with 30˚C).
The increase in IVPDP express an improvement of protein quality of M. oleifera leaves powder during the fermentation. The increase in IVPDP would be due to the drop in ANF contents [4] in particular phytates and to the increase in the protein content. The phytates are responsible to formation of the insoluble complexes with the nutrients such as proteins and minerals [61]. The hydrolysis of the phytates releases proteins from complex and increases their bioavailability.
Indeed, there is a positive correlation (p < 0.05) between the protein content (r = 0.42) and IVPDP, but negative correlation between the phytates content (r = −0.46) and IVPDP.
The increase in IVPDP can also result to hydrolysis of proteins to peptide and amino acids by L. plantarum A6 used as starter.
3.2.7. Iron Availability
The fluctuation of iron availability in the M. oleifera leaves powders during fermentation is presented by Figure 7. There is a general increase of iron availability during fermentation. This increase varies from 21.80% to 31.12% and 28.28% in the young leaves powders respectively fermented at 37˚C and 30˚C. In old-leaves powders, availability of iron increase from 31.86% to 52.14% and

Figure 7. Ion availability of M. oleifera fermented leaves powders (O37 = powders of 7-month-old leaves (old) fermented with 37˚C; O30 = powders of 7-month-old leaves (old) fermented with 30˚C, Y37 = powders of leaves of 1 month (young leaves) fermented with 37˚C; Y30 = powders of leaves of 1 month (young leaves) fermented with 30˚C).
47.94% for fermentations at 37˚C and 30˚C respectively. There is a positive correlation (p < 0.05) between time (r = 0.68) the temperature of fermentation (r = 0.52) and iron availability. The increase of iron availability can result to the reduction of phytates content. Indeed, phytic acid is responsible for the reduction of the availability of cations in food, forming insoluble complexes [4]. The hydrolysis of phytic acid release ions thus, allows an increase of minerals availability [59]. There is a negative correlation (p < 0.05) between phytates content (r = −0.73) and iron availability.
Fermentation is known as a process improving iron bioavailability in food of vegetable origin [59,61]. Several authors reported the effect of the reduction of phytates on the increase in the bioavailability of iron. IcardVernière et al. [60] noticed an increase of 31% of the soluble iron rate in the fermented millet with a reduction of almost 95% of phytates content. In the same way, an increase of iron bioavailable in fermented sorghum of 3.1% was reported by Towo et al. [58] during fermentation with a reduction of 88% of the content of phytates.
4. Conclusion
The fermentation of the M. oleifera leaves powders by Lactobacillus plantarum A6 reduce the phytates content to 66.92%, increase protein content and their peptic digestibility to a value of 63.97%. Fermentation also increases iron availability in these powders. The fermentation of M. oleifera leaves powders allows the improvement of its nutritional qualities. From this work, for the best improvement of nutritional qualities of M. oleifera leaf powder, the old leaves must be fermented during 120 hours at 37˚C. These fermented powders thus could be used to fight deficiency malnutrition, a public endemic health problem in the developing countries.
REFERENCES
- R. Bessah and A. Touzi, “Production de Protéines d’Organismes Unicellulaires (P. O. U) à partir des déchets de dattes,” Revue Energie Renouvelable, 2001, pp. 37-40.
- T. O. Akande and A. A. Odunsi, “Nutritive Value and Biochemical Changes in Broiler Chickens Fed Detoxified Castor Kernel Cake Based Diets,” African Journal of Biotechnology, Vol. 11, No. 12, 2012, pp. 2904-2911. doi:10.5897/AJB11.677
- K. L. B. Mezajoug, “Propriétés Nutritionnelles et Fonctionnelles des Proteines de Tourteaux, de Concentrats et d‟isolats de Ricinodendron heudelotii (Bail.) Pierre ex Pax et de Tetracarpidium conophorum (Müll. Arg),” Ph.D. Dissertation, National School of Agro-Industrial Sciences, University of Ngaoundéré, Ngaoundéré, 2010, 170p.
- I. Lestienne, “Contribution à L’étude de la Biodisponibilité du fer et du zinc dans le Grain de mil et Conditions D’amélioration dans les Aliments de Complément,” Thesis of Doctorat, Ecole doctorale des Sciences et Procédés Biologiques et Industriels Université, Montpellier, 2004, 224p.
- M. T. Ruel and M. Harimond, “Diversification Alimentaire, Couverture des Besoins Nutritionnels et Croissance des Enfants: Connaissances Actuelles et Recherches Nécessaires,” 2nd International Workshop Food-Based Approaches for a Healthy Nutrition: The Role of Food Technologists and Nutritionists, Ouagadougou, 23-28 November 2003.
- Dolcas Biotech, “Moringa oleifera,” 2009. http://www.dolcas-biotech.com/pdf/Moringa.pdf
- M. Ndong, S. Wade, N. Dossou, T. G. Amadou and R. D. Gning, “Valeur Nutritionnelle du Moringa oleifera, étude de la Biodisponibilite du fer, Effet de l’enrichissement de Divers Plats Traditionnels Sénégalais Avec la Poudre des Feuilles,” African Journal of Food Agriculture, Nutrition and Development, Vol. 7, No. 3, 2007, pp. 1-17.
- J. W. Fahey, “Moringa oleifera: A Review of the Medical Evidence for Its Nutritional, Therapeutic, and Prophylactic Properties: Part 1,” Trees for Life Journal, Vol. 1, No. 5, 2005, pp. 1-15. http://www.tfljournal.org/article.php/20051201124931586
- R. D. Gning, M. Ndong, S. Wade, N. Dossou and A. T. Guiro, “Valeur Nutritionnelle du Moringa oleifera, étude de la Biodisponibilite du fer, Effet de L’enrichissement de Divers Plats Traditionnels Sénégalais avec la Poudre des Feuilles,” African Journal of Food Agriculture, Nutrition and Development, Vol. 7, No. 3, 2007, pp. 1-17.
- L. L. Niba. “The Relevance of Biotechnology in the Development of Functional Foods for Improved Nutritional and Health Quality in Developing Countries,” African Journal of Biotechnology, Vol. 2, No. 12, 2003. pp. 631- 635.
- R. Shafiur, “Handbook of Food Preservation,” 2nd Edition, Taylor and Francis Group, London, 2007, p. 215. doi:10.1201/9781420017373
- A. O. Olusegun and G. N. Iniobong, “Spoilage and Preservation of Meat: A General Appraisal and Potential of Lactic Acid Bacteria as Biological Preservatives,” International Journal of Biotechnology, Vol. 2, 2011, pp. 33- 46.
- D. Louembé, S. C. Kobawila, G. K. Bouanga and S. Kéléké, “Etude Microbiologique des Feuilles Fermentées de Manioc: ‘Ntoba Mbodi’,” Tropicultura, Vol. 21, No. 3, 2003, pp. 106-111.
- A. A. Yao, M. Egounlety, L. P. Kouame and P. Thonart, “Les Bactéries Lactiques dans les Aliments ou Boissons Amylacés et Fermentés de l’Afrique de l’Ouest: Leur Utilisation Actuelle,” Annale de Médécine Vétérinaire, Vol. 153, 2009, pp. 54-65.
- M. Zamudio, A. Gonzalez and J. A. Medina, “Lactobacillus plantarum Phytase Activity Is Due to Non-Specific Acid Phosphatase,” Letters in Applied Microbiology, Vol. 32, No. 3, 2001, pp. 181-184. doi:10.1046/j.1472-765x.2001.00890.x
- E. Giraud, L. Gosselin, B. Marin, J. L. Parada and M. Raimbault, “Purification and Characterization of an Extracellular Amylase from Lactobacillus plantarum Strain A6,” Journal of Applied Bacteriology, Vol. 75, No. 3, 1993, pp. 276-282. doi:10.1111/j.1365-2672.1993.tb02777.x
- J. A. Perumal and J. Kadirvelu, “Screening of Lactobacillus plantarum Isolated from Fermented Idli Batter for Probiotic Properties,” African Journal of Biotechnology, Vol. 11, No. 65, 2012, pp. 12856-12864.
- D. R. Djoulde, F. X. Etoa, N. J. J. Essia and C. M. F. Mbofung, “Fermentation du Manioc Cyanogène par une Culture Mixte de Lactobacillus plantarum et Rhizopus oryzae,” African Journal of Microbiology Research, Vol. 5, No. 27, 2003, pp. 4866-4872.
- I. A. Adetunde, A. A. Onilude and L. A. Adetunde, “Effect of Particulate Materials on Lactic Fermentation of New Local White Variety Cassava (‘Bianbasse’) Using Both Spontaneous and Starter Culture,” African Journal of Microbiology Research, Vol. 4, No. 1, 2010, pp. 45-50.
- A. Mami, A. R. Hamedi, J. E. Henni, A. Kerfouf and M. Kihal, “Activité antibactérienne de Lactobacillus plantarum isolée du lait cru de chèvre d’Algérie vis à vis de Staphylococcus aureus,” Les Technologies De Laboratoire, Vol. 5, No. 21, 2010, pp. 26-31.
- L. T. Songré-Ouattara, C. Mouquet-Rivier, C. Icardvernière, I. Rochette, B. Diawara and J. P. Guyot, “Potential of Amylolytic Lactic Acid Bacteria to Replace the Use of Malt for Partial Starch Hydrolysis to Produce African Fermented Pearl Millet Gruel Fortified with Groundnut,” International Journal of Food Microbiology, Vol. 130, 2009, pp. 258-264.
- Association of Officials Analytical Chemist (AOAC), “Officials Methods of Analysis,” 13th Edition, Association of Official Analytical Chemist, Washington DC, 1980.
- Association of Officials Analytical Chemist (AOAC), “Officials Methods of Analysis,” 15th Edition, Association of Official Analytical Chemist, Washington DC, 1990.
- E. H. Fischer and E. A. Stein, “DNS Colorimetric Determination of Available Carbohydrates in Foods,” Biochemical Preparations, Vol. 8, 1961, pp. 30-37.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, “Colorimetric Method for Determination of Sugars and Related Substances,” Analytical Chemistry, Vol. 28, No. 3, 1956, pp. 350-356. doi:10.1021/ac60111a017
- M. B. Devani, C. J. Shishoo, S. A. Shah and B. N. Suhagia, “Spectrophotometric Method for the Determination of Nitrogen in Kjeldahl Digest,” Journal of Analitycal Chemistry, Vol. 72, 1989, pp. 953-956.
- J. Bourely, “Observation sur le Dosage de l’Huile des Graines de Cotonnier,” Coton et Fibres Tropicales, Vol. 27, No. 2, 1982, pp. 183-196.
- J. Rodier, “L’analyse de l’Eau: Chimie, Physico-Chimie, Bactériologique, Biologie,” 6th Edition, Dunod Technique, Paris, 1978, 1136p.
- I. S. Abiodun, A. O. Anthony and T. I. Omolara, “Biochemical Composition of Infant Weaning Food Fabricated from Fermented Blends of Cereal and Soybean,” Food Chemistry, Vol. 65, No. 1, 1999, pp. 35-39. doi:10.1016/S0308-8146(98)00132-0
- G. Marigo, “Méthode de Fractionnement et d’Estimation des Composes Phénoliques Chez les Végétaux,” Analysis, Vol. 2, No. 2, 1973, pp. 106-110.
- E. T. Mertz, M. M. Hasen, C. Cairns-Whittern, A. W. Kirleis, L. Tu and J. D. Axtell, “Pepsin Digestibility of Proteins in Sorghum and Other Major Cereals (Improved Pepsin Assay/Processing Effects/Temperature Effects),” Proceedings of the National Academy of Sciences of the USA, Vol. 81, No. 1, 1984, pp. 1-12. doi:10.1073/pnas.81.1.1
- O. R. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, “Protein Measurement with the Folin Phenol Reagent,” Journal of Biology and Chemistry, Vol. 193, No. 1, 1951, pp. 265-269.
- Statsoft, “Statistica for Windows,” SPSS Inc., Chicago, 1995.
- American Association of Cereal Chemist, “Approved methods of the American Association of Cereal Chemist,” AACC, St. Paul, 2000.
- Y. Ray-Yu, C. Lien-Chung, H. Jenn-Chung, B. C. W. Brian, C. P. Manuel, M. L. Chadha and V. Levasseur, “Nutritional and Functional Properties of Moringa Leaves—From Germplasm, to Plant, to Food, to Health,” Moringa and Other Highly Nutritious Plant Resources: Strategies, Standards and Markets for a Better Impact on Nutrition in Africa, Accra, 16-18 November 2006, pp. 1-9.
- J. Pallavi and M. Dipika, “Effect of Dehydration on the Nutritive Value of Drumstick Leaves,” Journal of Metabolomics and Systems Biology, Vol. 1, No. 1, 2010, pp. 5-9.
- B. Moyo, P. J. Masika, A. Hugo and V. Muchenje, “Nutritional Characterization of Moringa (Moringa oleifera Lam.) Leaves,” African Journal of Biotechnology, Vol. 10, No. 60, 2011, pp. 12925-12933.
- C. Tchiégang and K. Aissatou, “Données Ethnonutritionnelles et Caractéristiques Physico-Chimiques des Légumes-Feuilles Consommés dans la Savane de L’Adamaoua (Cameroun),” Tropicultura, Vol. 22, No. 1, 2004, pp. 11-18.
- D. M. Price, “The Moringa Tree,” 2007. http://www.echonet.org
- M. Broin, “Composition Nutritionnelle Des Feuilles de Moringa oleifera,” 2005. http://www.moringanews. org
- N. Richter, P. Siddhuraju and K. Becker, “Evaluation of Nutritional Quality of Moringa (Moringa oleifera Lam.) Leaves as an Alternative Protein Source for Nile Tilapia (Oreochromis niloticus L.),” Aquaculture, Vol. 217, No. 1-4, 2003, pp. 599-611. doi:10.1016/S0044-8486(02)00497-0
- N. Foidl, H. P. Makkar and K. Becker, “The Potential of M. oleifera for Agricultural Industrial Uses,” In: L. J. Fuglie, Eds., The Miracle Tree: Multiple Attributes of Moringa, CTA/CWS, 2001, pp. 45-76.
- N. K. Amaglo, G. M. Timpo, W. O. Ellis and R. N. Bennett, “Effet de L’écartement et la Fréquence des Récoltes sur la Croissance et le Rendement en Feuilles de Moringa oleifera Lam,” Moringa et Autres Végétaux à Fort Potentiel Nutritionnel: Stratégies, Normes et Marchés Pour un Meilleur Impact sur la Nutrition en Afrique, Atelier International, Accra, 2006, pp. 1-8.
- S. Bassa, L. Michodjèhoun-Mestres, V. Anihouvi and J. Hounhouigan, “Prévention de l’Anémie dans les Zones Rurales au Bénin: Aspects Technologiques de la Fortification en fer de la Farine Fermentée de Maïs,” 2nd International Workshop Food-Based Approaches for a Healthy Nutrition, Ouagadougou, 2003, pp. 577-588.
- S. Sefa-Dedeh, Y. Kluvitse and O. E. Afoakwa, “Influence of Fermentation and Cowpea Steaming on Some Quality Characteristics of Maize-Cowpea Blends,” African Journal of Science and Technology, Vol. 2, No. 2, 2001, pp. 71-80.
- B. O. Omafuvbe, “Effect of Salt on the Fermentation of Soybean (Glycine max) into daddawa Using Bacillus subtilis as Starter Culture,” African Journal of Biotechnology, Vol. 5, No. 10, 2006, pp. 1001-1005.
- M .E. El Hag, A. H. El Tinay and N. E. Yousif, “Effect of Fermentation and Dehulling on Starch, Total Polyphenols, Phytic Acid Content and in Vitro Protein Digestibility of Pearl Millet,” Food Chemistry, Vol. 77, No. 2, 2002, pp. 193-196. doi:10.1016/S0308-8146(01)00336-3
- A. E. A. Yagoub, B. E. Mohamed, A. H. R. Ahmed and A. H. El Tinay, “Study on Furundu, a Traditional Sudanese Fermented Roselle (Hibiscus sabdarifa) Seed: Effect on in Vitro Digestibility, Chemical Composition and Functional Properties of the Total Proteins,” Journal of Agriculture and Chemistry, Vol. 52, No. 20, 2004, pp. 6143- 6150. doi:10.1021/jf0496548
- D. R. Djoulde, “Mise au Point D’un Ferment Mixte Destine a la Bioconversion Des Tubercules de Manioc Cyanogène,” Ph.D. Dissertation, National School of AgroIndustrial Sciences, Ngaoundere University, Ngaoundere, 2005.
- D. R. Djoulde, N. J. J. Essia and F. X. Etoa, “Cassava Solid-State Fermentation with a Starter Culture of Lactobacillus plantarum and Rhyzopus oryzae for Cellulase Production,” African Journal of Microbiology Research, Vol. 5, No. 27, 2011, pp. 4866-4872.
- N. J. M Medoua, “Potentiels Nutritionnel et Technologique Des Tubercules Durcis de L’igname, Dioscorea dumetorum (Kunth) Pax: étude du Durcissement PostRécolte et Des Conditions de Transformation Des Tubercules Durcis en Farine,” Ph.D. Dissertation, National School of Agro-Industrial Sciences, Ngaoundere University, Ngaoundere, 2005.
- F. S. Ibrahim, E. E. Babiker, N. E. Yousif and A. H. El Tinay, “Effect of Fermentation on Biochemical and Sensory Characteristics of Sorghum Flour Supplemented with Whey Protein,” Food Chemistry, Vol. 92, No. 2, 2005, pp. 285-292. doi:10.1016/j.foodchem.2004.07.024
- P. Azokpota, D. J. Hounhouigan and M. C. Nago, “Microbiological and Chemical Changes during the Fermentation of African Locust Bean (Parkia biglobosa) to Produce Afitin, Iru and Sonru, Three Traditional Condiments Produced in Benin,” International Journal of Food Microbiology, Vol. 107, No. 3, 2006, pp. 304-309. doi:10.1016/j.ijfoodmicro.2005.10.026
- T. I. Mbata, M. J. Ikenebomeh and S. Ezeibe, “Evaluation of Mineral Content and Functional Properties of Fermented Maize (Zea mays) Flour Blended with Bambara Groundnut (Vigna subterranean L.),” African Journal of Food Science, Vol. 13 No. 4, 2009, pp. 107-112.
- B. Opere, O. O. Aboaba, E. O. Ugoji and B. A. Iwalokun, “Estimation of Nutritive Value, Organoleptic Properties and Consumer Acceptability of Fermented Cereal Gruel (OGI),” Advance Journal of Food Science and Technology, Vol. 4, No. 1, 2012, pp. 1-8.
- M. R. Mona, E. M. Abeer, M. A. Hala and U. N. Mohamed, “Improvement of Nutritional Quality and Antioxidant Activities of Yeast Fermented Soybean Curd Residue,” African Journal of Biotechnology, Vol. 10, No. 28, 2011, pp. 5504-5513.
- N. L. Tatsadjieu, F.-X. Etoa and C. M. F. Mbofung, “Drying Kinetics, Physico-Chemical and Nutritional Characteristics of ‘Kindimu’, a Fermented Milk-Based-Sorghum-Flour,” The Journal of Food Technology in Africa, Vol. 9, No. 1, 2004, pp. 17-22.
- E. Towo, E. Matuschek and U. Svanberg, “Fermentation and Enzyme Treatment of Tannin Sorghum Gruels: Effects on Phenolic Compounds, Phytate and in Vitro Accessible Iron,” Food Chemistry, Vol. 94, No. 3, 2006, pp. 369-376. doi:10.1016/j.foodchem.2004.11.027
- M. O. Nuha, A. M. A. Isam and E. B Elfadil, “Chemical Composition, Antinutrients and Extractable Minerals of Sicklepod (Cassia obtusifolia) Leaves as Influenced by Fermentation and Cooking,” International Food Research Journal, Vol. 17, 2010, pp. 775-785.
- C. Icard-Vernière, V. Greffeuille, B. Caporiccio, S. Trèche and P. Besançon, “Influence du Trempage, de la Germination, de la Fermentation et de l’Ajout de Phytases Sur la Biodis-Ponibilité du Fer et du Zinc dans les Farines de Mil,” 2nd International Workshop FoodBased-Approaches for a Healthy Nutrition Ouagadougou, 2003, pp. 427-436
- O. Nam-Soon and I. Man-Jin, “Phytate Degradation by Leuconostoc mesenteroides KC51 Cultivation in Soymilk,” African Journal of Biotechnology, Vol. 8, No. 13, 2009, pp. 3023-3026.
NOTES
*Corresponding author.

