Synthesis and Characterisation of Colorants Derived from 1,4-Diamino Anthraquinone Polyamides ()
1. Introduction
Study on the synthesis and application of polymeric dyes has aroused interest in recent years. Polymeric dyes due to the advantage of molecular size do not sublime, are nonabrasive, and generally have low toxicity [1]. They have been widely applied on fibres because of certain essential dye properties such as fast levelling, fastness to light and wet treatment, low sublimation rate, and very good thermal and chemical resistance [2,3]. Xu et al., [4] stated that polymeric dyes have excellent brightness and are extremely fadeless. They have been used in food coloration and surface coating of plastic objects, as hair dyes [5]; solid state laser dyes [6]; jet Printing [1,7].
Polymeric dyes constituting polyesters, polyurathanes and polystyrene have been reported several years ago [8-10]. Research activity in the area is still very active; polymeric dyes containing the anthraquinone structure and their application on polyethylene terephthalate [11] (Qinghua, 2002), synthesis and light emitting properties of polymeric metal complex dyes based on hydroxyquinoline moiety [12]; polymeric dyes for photovoltaic applications [13]; and synthesis of novel water soluble cross linked polymeric dye with good dyeing properties [14]. Others include the synthesis of coloured polyureas [15,16], polyurethane derivatives polymeric dyes that have fluorescent properties have also been reported [17, 18].
The paper discusses the synthesis of polymeric dyes using low temperature solution polycondendensation technique and characterizing the products using FT-IR spectrophotometry, differential Scanning Calorimetry and Thermogravimetry. Attempt has been made to apply the dyes unto cotton fabric; a cellulosic material by leuco vatting method. This is the first attempt to do this as no literature source has hinted to this. The dyes were also applied to polyester materials by high pressure as disperse dyes.
2. Experimental
2.1. Reagents
Anthraquinone, 1,4-diamino, was purchased from Sigma Aldrich and was used without further purification. Malonic acid (BDH), Succinic acid (BDH), Adipic acid (BDH), thionyl chloride, ethanol, chloroform, petroleum ether, sodium hydroxide, DMF and methanol are reagent grade and were used without further purification.
2.2. Instruments
Melting points were determined with Gallenkamp melting point apparatus, intrinsic viscosities were measured in ethanol using Ubelohde viscometer, CECIL CE 7400 model spectrophotometer was used for UV/Visible spectrometry, FT-IR spectra were obtained using Fourier Transformed Infra red (FT-IR) spectrometer (Perkin Elmer Model), DSC studies were conducted using Dupont 200 thermal analyser.
2.3. Method for the Synthesis of Polymeric Dyes
Anthraquinone, 1,4-diamino (0.01 M) was dissolved in 15 ml of N,N-dimethylformamide in a 100 ml onenecked flask. Diacid halide (0.01 M) was added portion wise with stirring while the temperature was maintained at 15˚C in an ice-cooled water bath for one hour. The solution was then poured into a blender cup containing 200 ml water and vigorously blended to precipitate the polymer. The polymer was filtered, washed several times with water and dried in an oven at 100˚C for 15minutes [18-20].
2.4. Methods for the Characterisation of the Polymeric Dyes
2.4.1. FT-IR Spectroscopy
The samples were characterized using KBr disc sampling method. The disks were prepared by grinding the sample (2% by weight) in KBr which was then compressed into a disk and analyzed with the spectrophotometer. All the Spectra were recorded over the range 4000 - 400 cm−1. [21].
2.4.2. UV/Visible Spectroscopy
UV/Visible spectroscopic characterization was done with a CECIL CE 7400 Spectrophotometer. The samples were dissolved in ethanol to give a 0.01 g/dm3 solution.
2.4.3. Differential Scanning Calorimetry (DSC)
A Dupont 200 thermal analyser was used to study the thermal properties of the polymeric dye samples in aluminium pans. The calorimeter was used with nitrogen as carrier gas, heating from 28˚C to 308˚C at a rate of 10˚C per minute as described by previous workers [22,23].
2.4.4. Thermogravimetric Analysis (TGA)
Thermogravimetric analysis (TGA) of approximately 200 mg dried samples was carried out at a heating rate of 10˚C·min−1 from room temperature to 700˚C under a nitrogen atmosphere on a TG-IR interface [24].
2.5. Application of the Dyes
2.5.1. Dyeing of Cotton Piece
A dye bath was prepared by dissolving 0.5 g of the powder in 5 ml of ethanol and made 100 ml with distil water. To this dispersion, 5 ml Caustic soda solution (10%) and 0.1 g sodium dithionite were added. The solution was heated at 60˚C until a clear solution was obtained. A cotton fabric, 0.5 g was then placed into the dyebath and dyeing carried out at 50 ˚C - 60˚C for about 45 min. The fabric was then removed, squeezed without rinsing and allowed to stand in 10 min in the open air for oxidation. This is followed by rinsing in dilute acetic acid, hot water and then boiled in soap solution for 15 min and finally thoroughly rinsed in running tap and dried.
2.5.2. Pressure Dyeing of Polyester
A suspension of the dye was made involving 0.5 g of the dyes in ethanol and water as above and poured into a pressure cooker. A polyester fabric sample, 0.5 g was placed into the cooker and dyed for 1hour with continuous agitation. The sample was then removed, rinsed with detergent, water and then dried.
2.5.3. Properties of the Dyed Samples
Both fabric samples were tested for wash, light and rubbing fastness.
3. Results and Discussion
3.1. Synthesis of the Polymeric Dyes
The reaction between the diacid halides and the diamine anthraquinone leads to the polyamides according to Scheme 1, pale to bluish purple precipitates are produced. The aromatic diamine has low basicity due to the delocalization of the lone pairs on the amine groups leading to conjugation with the extended ring system. As a result of this its reactivity has to be catalysed by the addition of N,N-dimethylformamide which served both as a solvent and a proton acceptor. The percentage yields of the products being 63.4%, 61% and 54.5% for PolyNAM, PolyNAS and PolyNAA respectively contrasted significantly with the over 95% yield reported by Joshi et al., [16]; in the synthesis of coloured polyureas. Though the low reactivity of the aromatic diamines could account for these poor yields, some of the dyes could also have been lost during isolation of the products from the solvent system used.
3.2. Characterization
3.2.1. Structure Confirmation by FT-IR Spectroscopy
The IR-spectra of the samples; summarized in Table 1 are identical in most respects; this is mainly because the polymers are homologues differing in the molecular formula of the repeat units by a -CH2- [25]. They all show weak bands at 3387 - 3392 cm−1 due to the amide-N-H stretching [26,27]. The peaks at 3263 - 3265 cm−1 are attributable to N-H absorptions of the end
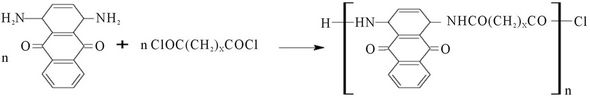
Key Monomer Polymer Code X = 1
Malonylchloride Poly(N-anthraquinonylmalonamide) PolyNAM X-2
Succinoylchloride Poly(N-anthraquinonylsuccinamide) PolyNAS X = 3
Adipoylchloride Poly(N-anthraquinonyladiponamide) PolyNAA
Scheme 1. Schematic illustration of the polymeric dye synthesis.

Table 1. Infra red absorption bands (Ibrahim, 2010).
groups. The peaks at 1565 - 1566 cm−1 are due to C=O stretching of the amide linkage and the peak at 1440 cm−1 is ascribed to the anthraquinone C=O absorption. These double bands are characteristic of amides [27-29]. The shift in the position of the absorption bands to lower frequencies could be due to the presence of H-bonding in the compounds [26].
3.2.2. Melting Point of the Polymeric Dyes
As seen in Table 2; all the three polymeric dye products are found to melt between 265˚C - 272˚C. The very close range of melting temperatures may be attributed to the similarity in their chemical structures. However, the little variations could be due to the variation in molar mass of the repeat units and the molecular mass of the polymers [27].
3.2.3. Solubility of the Polymeric Dyes
The solubility properties of the polymeric dyes are summarized in Table 3.The products are not soluble in most of the solvents used for the test. Solubility of the polymeric dyes in polar organic solvents could be attributed to the presence of polar N-H bond. The slight solubility in water is also attributable to hydrogen bonding between the amine/amide groups in the dye and hydroxyl groups in water [27].
3.2.4. Viscosity of the Polymeric Dyes
The viscosities of the polymeric dyes at 25˚C are presented in Figure 1. The polymers indicated low intrinsic

Table 2. Melting points of the dye samples.
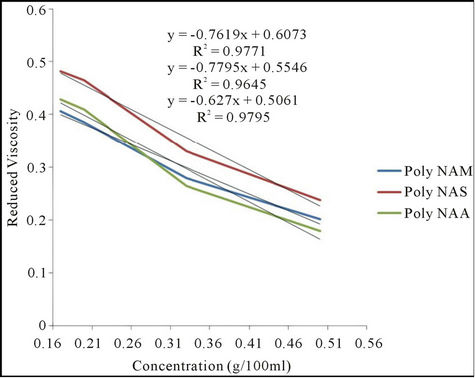
Figure 1. Plot of reduced viscosity against concentration.
viscosity which implies low molecular weight. By this property, the polymeric dyes will have a better ability of penetrating the fiber more than if the molecular weight were very high. The result agrees with the literature reported [11].
3.2.5. UV/Visible Spectroscopy
The analysis was carried out to determine the wavelength of maximum absorption (λmax) for the polymeric dye samples. Results obtained are as shown in Table 4 and Figure 2.
The colour of the parent anthraquinone (1,4-diamino anthraquinone) was violet, with maximum absorption wavelength (λmax) of 550nm. But after condensation, the colours of the polymeric dyes deepened to bluish violet all indicating bathochromic shifts of between 39 - 52 nm. The absorptivity coefficients (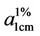 ) of the polymeric dyes were all extremely higher than that of the parent
) of the polymeric dyes were all extremely higher than that of the parent

Table 3. Results of solubility test.
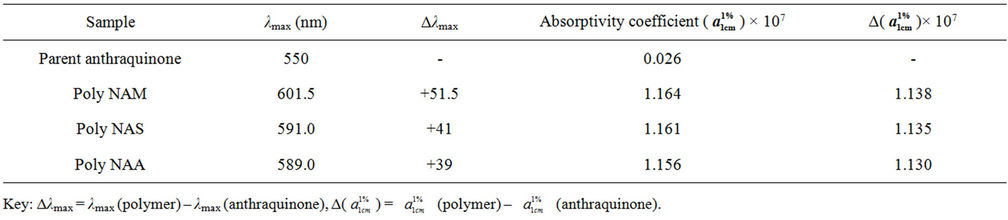
Table 4. λmax Values for the dye samples.
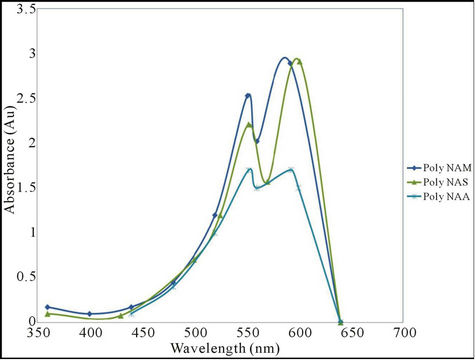
Figure 2. UV Visible Spectra of the polymeric dyes.
anthraquinone ie hyper chromic shifts of about 1.13 × 107. This result is consistent with an earlier work [30]. However both parameters suggested that the higher the molecular weight of the repeat unit the lower were the shifts.
3.2.6. Differential Scanning Calorimetry
Exothermic and Endothermic temperature values are presented in Table 5. Typical DSC spectra are presented on Figures 3-5. Differential Scanning Calorimetry (DSC) revealed a high melting exotherm at 270˚C, 267˚C and 269˚C for PolyNAM, PolyNAS and PolyNAA respectively. The values are very close consistent with the basic structures of the monomers. PolyNAM and PolyNAS show Tg at 50˚C and 55˚C respectively. PolyNAA shows an endothermic peak at around 182˚C, this is considered the crystallization temperature. It is evident from the DSC thermograms that only PolyNAA shows the ability to crystallize. This could be due to a number of factors such as average chain length, degree of polymerization, intermolecular forces of attraction etc. [28,31,32].
3.2.7. Thermogravimetric Analysis
The thermograms of the samples are indicated in Figures 6-8 and Table 6. Thermogravimetric analysis indicated that both polymers release some low molecular weight molecules probably water, at the peak temperatures of 60˚C, 64˚C and 85˚C respectively for PolyNAM, PolyNAS and PolyNAA. In addition to the trend of increasing peak temperature with molecular weight of the diacids, the weight loss however decreased in the order 2.86%, 1.67% and 1.28% respectively. PolyNAM and PolyNAS recorded weight losses of 5.9% (onset 123˚C) and 7.1% (onset 198˚C) respectively all with upper limit of 285˚C. PolyNAA which recorded a weight loss of 61% at 374˚C indicated an onset of 229˚C. It is very clear that both Polymers have identical thermal stabilities loosing less than 10% of weight at 300˚C. The thermal stabilities of these polymers are however lower than Polyureas reported by Jauhari et al. [16].
No work seems to have been done on the application of carbonyl base polymeric dyes to cellulosic materials by vatting method. Majority of polymeric dyes synthesized are applied as disperse dyes to mostly hydrophobic substrates [33]. Some dyeing properties given in Table 7 showed that all the dyes have excellent washing and rubbing fastness on the two sample types and very good light fastness ratings. The excellent wash fastness recorded agreed with the findings of several workers [2, 30].
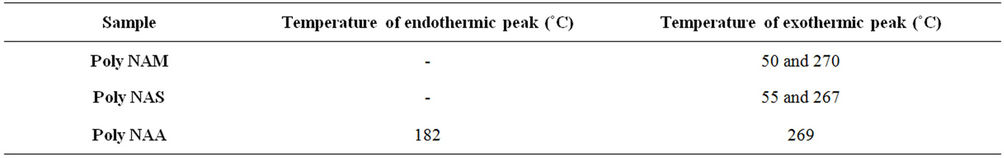
Table 5. DSC endothermic/exothermic peaks for the dye samples.
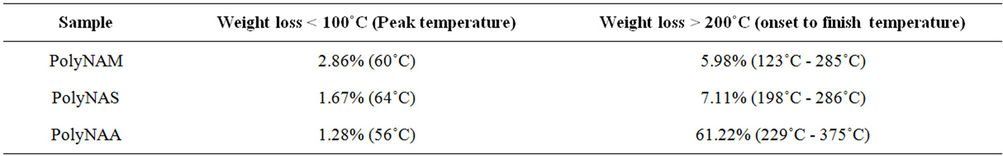
Table 6. TGA selected weight loss data.
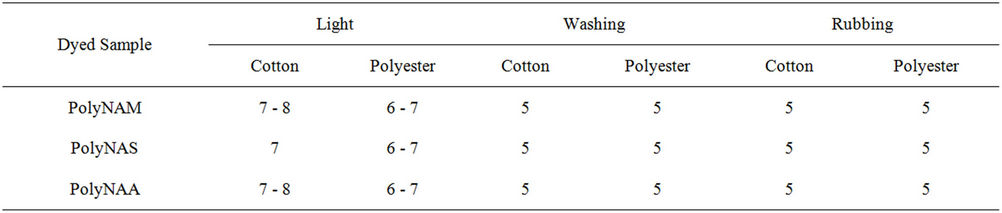
Table 7. Dyeing properties of the dyes on cotton and polyester fabrics.
4. Conclusion
In this work three polymeric dyes containing anthraquinone chromophore were synthesized. The synthesis was achieved using low temperature solution polycondensation technique. Formation of the amide polymeric dyes was confirmed using FT-IR spectroscopy. The products were observed to possess high absorptivity coefficient. Differential Scanning Calorimetry and Thermogravimetry showed that the dyes have good thermal stabilities.
5. Acknowledgements
The authors wish to acknowledge the contribution of Dr Abdulsalam A. Salisu and Department of Chemistry, Universiti Putra, Malaysia, for conducting the TGA and DSC analysis and providing the TGA and DSC spectra presented in this write up.
NOTES