Effect of Reaction Conditions on Production of Catechinone Hair Dyestuff in Water/Alcohol Mixed Solution ()
1. Introduction
A great number of people dye their hair in the world today. Above all, human hair dyeing by using oxidation dyes is most frequently employed. The advantages of the permanent hair colouring technique using the oxidation dye are the higher dyeability for darker hair and higher colour fastness to washing [1] [2] . However, many kinds of symptoms are caused for some people after using of the oxidation hair dyes (For example, [3] [4] ). The problems should be solved for better human life also by scientific way.
Under such the situation, the authors invented catechinone (4-(3,4-dihydro-3α,5,7-trihydroxy-2H-1-benzopyran-2α-yl) 1,2-benzoquinone) as a safer hair dyestuff and it is prepared by oxidising (+)-catechin [5] . The obtained colourant from (+)-catechin contains a small amount of byproducts but the main product is catechinone, of which structure is shown in Figure 1. Catechinone dyes human hair orange, reddish orange and deep yellowish brown. The colour fastness of the dyed hair to washing and light is high enough for practical use. Catechinone does not cause erythema or oedema on skin of rabbits. The dyestuff is obtained by enzymatic [5] and chemical [6] oxidation methods. It is produced by using a catechol oxidase such as tyrosinase and ascorbic acid oxidase, and the dye formation rate and the reaction selectivity of the enzymatic reactions are high. On the other hand, it is prepared by using O2 gas in basic solution, where the formation rate is lower than that of the enzymatic method. However, the chemical method is easier to control and useful industrially because of the low cost. The catechinone formation occurs via proton dissociations and one electron oxidations by oxygen, thus the production efficiency of the dyestuff is improved by increasing pH of aqueous solution and the concentration of O2 [6] .
Nevertheless, the yield of catechinone is not high in one batch of the reaction solution because of the low solubility of (+)-catechin in an aqueous solution, and then it should be improved for practical use. The solubility of (+)-catechin in ethanol is over 30 times higher than that in water. Then, the chemical dyestuff preparation was attempted by adding ethanol into the reaction solution and increasing the concentration of (+)-catechin to increase the dye production efficiency in the previous study [7] . The results showed that catechinone is not formed in pure ethanol solution but it is obtained in the water/ethanol mixed solution at 0.28 of the ethanol molar fraction of the solvent. The amount of the dyestuff formed in water/ethanol mixed solution is over 20 times larger than that in the aqueous solution system. It was suggested that water-ethanol mixing ratio of the solvent is an important factor for the formation efficiency. The other reaction conditions such as basicity and temperature are considered to be the key factor for the formation, too. It is also interesting to investigate the availability of other water-soluble alcohols for the reaction and the effect of the alkyl group structure of the alcohol on it.
In this study, the relationships between the amount of the formed catechinone and the dye preparation conditions such as basicity, sorts of added base, temperature, alcohol molar fraction of solvent and sorts of added alcohols were studied for the oxidation reaction of (+)-catechin in water/alcohol solution.
2. Experimental
2.1. Materials
(+)-Catechin and other reagents described below were purchased from Sigma and Nacalai tesque, respectively. (+)-Catechin hydrate, ethanol (EtOH, 99.5%), methanol (MeOH, 99.8%), 1-propanol (1-PrOH, 99.5%), 2-propanol (2-PrOH, 99.7%) and tert-butyl alcohol (t-BuOH, 99.0%) were used without further purification. Monoethanol amine (MEA), diethanol amine (DEA), triethanol amine (TEA), l-arginine (Arg), sodium carbonate (Na2CO3), potassium carbonate (K2CO3), sodium phosphate (Na3PO4) and sodium hydroxide (NaOH) were used as base without further purification.

Figure 1. Chemical structure of catechinone.
2.2. Dyestuff Preparation
(+)-Catechin and a base were dissolved in 100 g of distilled water/alcohol mixed solvent. Oxygen gas (≥99.5 vol%) was introduced continuously into the reaction solution through porous glass ball filter (pore size: 40 - 50 μm) at 100 ml∙min−1 of flow rate, and the solution was stirred for prescribed time at 20˚C - 50˚C.
2.3. Measurements
The reaction solution was sampled at several time intervals and diluted by distilled water with a dilution ratio (f). The ultraviolet-visible absorption spectra of the sampled solution were measured by a Hitachi U-3900H spectrophotometer at 25˚C. The dye formation behaviour was monitored by using the absorbance at 430 nm (A430) at each reaction time (t). The obtained f·A430 values correspond to the concentration of the formed dye [7] and were used the index of the amount of the dyestuff formed. The dyestuff formation rate (v) was calculated by v = [Δ(f·A430)] max/Δt, where Δt is the reaction time interval and [Δ(f·A430)]max the maximum increment of f·A430 for the Δt. Constant Δt was adopted for the calculation of v for each the experiment.
The apparent basicity of the reaction mixed solution (pH*) was determined through the measurement by a TOA-DKK MM-60R multi water quality meter with a TOA-DKK ELP-031 glass combination electrode at 25˚C.
3. Results and Discussion
3.1. Effects of Basicity and Sorts of Bases
The colour of reaction solution turns orange and reddish brown with reaction time showing the formation of catechinone dyestuff. Catechinone is hardly obtained in acidic and neutral reaction solution without base. The rate of catechinone formation increases with increasing the basicity of aqueous reaction solution [6] and it is increased by using a stronger organic base [7] . The previous results indicate the strong influence of basicity on the reaction. Then, the effects of basicity and sort of bases on the catechinone formation were studied first. The pH is not defined in the water/organic solvent mixture, of which organic composition is high, and the apparent basicity was estimated as using pH* in the study. The amount of the formed dyestuff at 200 min of reaction time ((f·A430)200min) and the dyestuff formation rate (v) value in the water/ethanol mixed solution for each of the experiment with a variety of bases changing their concentration (molarity: mB) were measured and determined. The alcohol molar fraction of the mixed solvent (xA) was 0.28.
Figure 2 shows the relationship between pH* and (f·A430)200min for the water/ethanol solution with MEA. The resulting values are also summarised in Table1 The (f·A430)200min and v increase with increasing pH* and mB of MEA as shown in the figure and for experimental number 2 - 5 in the table. The results show that the reaction is
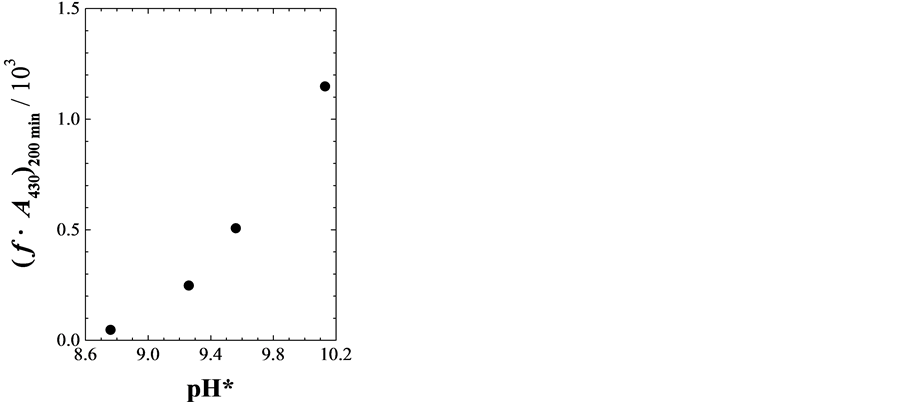
Figure 2. The pH* dependence of the (f·A430)200min for the catechinone produced in water/ethanol solution (xA = 0.28) with MEA at 30˚C.

Table 1. The reaction condition and the resulting rate and amount of catechinone dye. The reactions are started in water/ethanol solution (xA = 0.28) at mS = 0.172 mol∙kg−1 at 30˚C.
aPrevious work [7] ; bThe base dissociation constant in water at 25˚C [8] ; cApparent pH value in the water/ethanol mixed solvent; df·A430 at 80 min.
promoted by higher basicity of the reaction solution. This relationship is similar to that between pH and the rate of catechinone formation in aqueous solution [6] . The MEA is also more effective for the formation than DEA and TEA. The deprotonated (+)-catechin in a basic medium works as an electron donor and reacts with O2 easily [6] [9] . Therefore, the higher basicity should contribute to promote the formation reaction also in the water/ ethanol solution.
Furthermore, the preparation of the dyestuff was made in the mixed solution with basic amino acid l-arginine or inorganic bases such as Na2CO3, K2CO3, Na3PO4 or NaOH (as listed in Table 1). The solubility of Na3PO4 in the water/ethanol mixed solution is low and its saturated concentration is 0.0068 mol∙kg−1. The mB of other inorganic bases was 0.10 mol∙kg−1. The results in Table 1 show that f·A430 for the reaction system with Na2CO3, K2CO3 or NaOH is larger than that with same concentration of MEA. However, it is lower than that with 0.50 mol∙kg−1 of MEA, and the solubility of inorganic bases in the water/ethanol mixed solution is lower as compared with that of MEA. The solution pH* decreases sharply at the earlier stage of the reaction for the inorganic base systems. This results in the decrease of the reactivity. In contrast, the pH* decreases gradually in the MEA system. Consequently, using MEA as the base is of greater advantage for the production.
3.2. Effects of Temperature
The rate of oxidation reactions are generally influenced by temperature. It is instructive to study the effect of reaction temperature on catechinone formation for obtaining better condition to produce the dyestuff efficiently. Figure 3 indicates the relationship between the reaction temperature (T ) and f·A430 at 140 min ((f·A430)140min) for the catechinone preparation in water/ethanol solution (xA = 0.28) with MEA. Here, the values of f·A430 at 140 min are adopted, because f·A430 increases and then decreases with reaction time at higher temperature and the downward turn begins before 200 min as described at the next section. The amount of the formed dye increases with increasing T up to 30˚C and it decreases with increasing T over 30˚C. The formation of colourless products and/or the precipitation of colourants may be caused by the further reactions of (+)-catechin and catechinone, and such the side reactions give rise to the decrease in f·A430 at higher temperature [6] . In fact, the maximal value of f·A430 decreases with T. This indicates that higher temperature promotes the reactions too much. It can be said in conclusion that 30˚C is the most preferable for producing the dyestuff in the water/alcohol mixed solution.

Figure 3. Relationship between reaction temperature (T) and (f·A430)140min for catechin preparation in water/ethanol mixed solution with MEA at xA = 0.28.
3.3. Effect of Alcohol Content and Sorts of Alcohols
Figure 4 shows the time course of change in the amount of the formed dye (f·A430) for catechinone preparation in water/ethanol mixed solution with MEA. The xA was changed from 0.10 to 0.97. The dye formation behaviour strongly depends on the xA as shown in the figure. The f·A430 increases monotonously with t at xA = 0.10 - 0.20 and 0.69 - 0.97. On the other hand, f·A430 increases and then decreases with t at xA = 0.25 - 0.59. The maximal value of f·A430 also changes with xA of 0.25 - 0.59 and the maximum of f·A430 is given at xA = 0.25. The formation of colourless products and/or the precipitation of colourants as described above may be caused also here leading to the decrease in f·A430 at xA = 0.25 - 0.59.
Figure 5 depicts the relationship between xA and f·A430 obtained at 140 min ((f·A430)140min) in water/ethanol mixed solution with MEA. The reason why the value of f·A430 obtained at 140 min is also adopted here, is that the same experiments were made in water and other alcohols as described below and the reactivities can be compared by using (f·A430)140min. The (f·A430)140min increases steeply from xA = 0.10 to 0.25 and decreases from 0.28 to 0.97. The results show that the amount of formed dye depends strongly on the ethanol molar fraction and reaches its peak at xA = 0.25. The results are interesting and it is thought that the effect of alcohol content for the reaction consists of two factors at least such as promotion and depression. Further, the same experiments were carried out using other alcohols being miscible with water such as methanol, 1-propanol, 2-propanol and tert-butyl alcohol. The results exhibit that the catechinone can be produced efficiently by adding all the alcohols used here and time change of f·A430 for the alcohols is in a similar manner as in the water/ethanol solution. The f·A430 for each reaction in the water/alcohol solutions increases monotonously with time, or reaches a peak and decreases. The f·A430 value for water/1-propanol solution at xA = 0.15 takes a downward turn after 140 min as an earliest beginning of the decrease. Therefore, the value of (f·A430)140min is adopted in order to make better comparison of the whole results of f·A430 under increasing. Figure 6 shows the relationships between the alcohol molar fraction for each of the mixed solvents (xA) and (f·A430)140min for each of the reactions made in each water/ alcohol mixed solution. The (f·A430)140min for all the systems shows maximum against xA. The (f·A430)140min represents the maximal value at 0.45, 0.25, 0.20, 0.15 or 0.10 of xA for the MeOH, EtOH, 2-PrOH, 1-PrOH or tBuOH-mixed system, respectively. It is, then, important to clarify the basis for the order of the xA values. The change in (f·A430)140min as shown in Figure 6 might relate with the concentration of O2 in the solution, the polarity of the mixed solvent, and/or a particular microstructure formed in the reaction solution. The authors study the mechanism and the reason for obtaining the results as shown in Figure 5 and Figure 6 at present.
Next, the dependence of the maximal amount of formed dye on the concentration of (+)-catechin in the reaction solution with the alcohols was investigated to elucidate the optimum condition for producing the dyestuff. MeOH, EtOH and 1-PrOH are convenient linear alcohols and they were employed in the experiments as at each xA = 0.45, 0.25 or 0.15, respectively for obtaining maximum. Figure 7 exhibits the relationships between the concentration of (+)-catechin (molarity: mS) and the maximal amount of formed dye ((f·A430)max) in the three


Figure 5. Relationship between alcohol molar fraction (xA) of the water/ethanol mixed solvent and the amount of the catechinone at 140 min ((f·A430)140min).

Figure 6. Relationship between xA and (f·A430)140min for the dye production at 140 min with O2 gas introduced at 30˚C in the water/methanol (¢), water/ethanol (), water/1-propanol (r), water/2-propanol (s) or water/tert-butyl alcohol (¯) solution with MEA.

Figure 7. Relationship between maximum of f·A430 ((f·A430)max) and the concentration of (+)-catechin (mS) for the dye formation in water/alcohol mixed solution with MEA at 30˚C. The xA is 0.45 (¢), 0.25 () or 0.15 (r) for the methanol, ethanol or 1-propanol added system, respectively.
kinds of water/alcohol systems. The maximum value of f·A430 is ca. 1600 in all the reaction systems. The mS value to give the maximum f·A430 for water/EtOH and water/1-PrOH systems is 0.35 mol∙kg−1 and the values for water/MeOH system are 0.35 and 0.59 mol∙kg−1.
The results show that the amount of formed catechinone depends on the sort of alcohols, the alcohol molar fraction and the concentration of (+)-catechin, and there are optimum xA and mS values to obtain the dyestuff efficiently. It can be said that the EtOH is most preferable as the solvent added for the production of the dyestuff, because its safety is highest, its handling is easiest during and after the production and it is most economical.
4. Conclusion
The relationships between the amount of the formed catechinone and the dye preparation conditions such as basicity, sorts of added base, temperature, alcohol molar fraction of solvent and sorts of added alcohols were studied for the oxidation reaction of (+)-catechin in water/alcohol solution. The amount of catechinone obtained increases with increasing basicity and the dyestuff is obtained by adding MEA, DEA, TEA, l-arginine, Na2CO3, K2CO3, Na3PO4 or NaOH. The optimum temperature for the production in water/ethanol solution is 30˚C. The optimum alcohol molar fraction of the mixed solvent for the dye formation is 0.45, 0.25, 0.20, 0.15 or 0.10, with the methanol, ethanol, 2-propanol, 1-propanol or tert-butyl alcohol system, respectively. The amount of the obtained dyestuff reaches maximum at 1) 0.35 and 0.59, 2) 0.35 or 3) 0.35 mol∙kg−1 of the fed concentration of (+)-catechin for the 1) water/MeOH, 2) water/EtOH or 3) water/1-PrOH system, respectively.
Acknowledgements
This study was financially supported partly by the Japan Society for the Promotion of Science Research Foundation Grant (No. 21500732) and partly by Japan Science and Technology Agency as Adaptable & Seamless Technology Transfer Program through Target-driven R & D (No. AS2211611E). Japanese patent application number: 2012-219084 (12 November 2012).
NOTES
*Corresponding author.