Manifestation of Key Molecular Genetic Markers in Pharmacocorrection of Endogenous Iron Metabolism in MCF-7 and MCF-7/DDP Human Breast Cancer Cells ()
1. Introduction
A new round of research in the field of tumor cells’ biology significantly changed the common view not only about the latent period of malignancy, its clinical and morphological classification, characteristics of the proliferative and metastatic potential, but also the selection of treatment, the criteria for disease-free and overall patients’ survival.
If, for example, we consider only the cellular heterogeneity and microenvironment in tumor tissue, then modern immunohistochemical subtypes from basal and luminal A/B to “triple negative” breast cancer are just like surrogate analogues of real molecular genetic characteristics that can be significant for clinical oncology [1,2].
One of the basic reasons for the heterogeneity of tumor tissue is the genetic and genomic instability caused by DNA sequence violations and rearrangement of chromosomes that determine the level of oncogenes and expression of tumor suppressors [3,4].
Increased expression of certain genes and reduction of others constantly change their dynamic balance, which determines not only the degree of heterogeneity, but also the level of sensitivity to therapy [5].
The emergence of these changes is predominantly associated with violation of the program of epigenetic regulation of tumor cell, which is accompanied by general hypomethylation and hypermethylation of promoters of individual genes. The rapid progress in studying of epigenetic regulation mechanisms of gene expression revealed regulatory role of small miRNA molecules, which regulate post-translational process at the level of protein synthesis [6-8].
However, significant progress in understanding of the nature of the malignant process does not produce the expected results in terms of early diagnostics, effective treatment and life quality improvement of cancer patients.
The need to improve clinical outcomes dictates a paradigm shift in the system of accumulation, analysis and interpretations of our knowledge.
Recent fundamental studies on metabolism of endogenous and exogenous iron in normal and tumor cells showed high degree of Fe ions integration into biological systems of human organism that offered a prospect of new markers and targets identification for development of antineoplastic agents. Iron is an essential element for cell growth and division because it exists in the structure of active sites of various proteins and takes part in many different reactions. Iron ions property to change quickly its valence from Fe2+ to Fe3+ made them vitally important in such reactions as DNA synthesis, oxygen transport, energy metabolism, cell cycle control, etc. At the same time its high reactivity makes it rather toxic compound, which causes hydroxyl radicals production. H. Fenton showed that during interaction with different reductants inside, the cell Fe3+ turned into Fe2+ [9]. It is known that formed reactive oxygen species interact with lipids, proteins, DNA, causing mutations and cell damage, which can initiate malignant transformation. Such unfavorable scenario for normal cell might become an optimal finding for selective damage of tumor cell. One of the unique properties of tumor cells is their increased affinity to Fe2+ ions [10-12]. It is important that intracellular Fe depot exists in forms of free/bound iron pools and ferritin. In healthy adults about 25% - 30% of Fe ions are connected with ferritin, thus preventing the development of toxic effects caused by this metal [13]. In normal cells in cases of supplement needs, iron is escaped from depot and is transported out of the cells by ferroportin and serum transferrin [14]. High intracellular Fe level causes ferritin translation, while its deficiency blocks this process [15]. Due to potential toxicity and possibility of reactive oxygen species (ROS) generation, the Fe level in organism is strictly controlled [16]. Instead, this is not typical for tumor cell. For activation of DNA synthesis it elevates ferritin levels, including multidrug resistance (MDR) formation [12,17]. And this is the second paradox. The fact of immunological identity between ferritin and autocrine growth factor secreted by leucosis cells is very interesting [18].
Today it is well known that besides the role in DNA synthesis, Fe ions affect the expression of proteins that are involved in cell cycle regulation, cell proliferation and angiogenesis and participate in the formation of cells’ metastatic potential. Numerous studies have proved the role of iron in the anti-tumor effect of cytotoxics [19,20]. A new wave of interest in the investigation of Fe ions and iron-containing proteins greatly expanded understanding of their dominant role in vital functions of both normal and tumor cells [12]. Today, it is clearly possible to speak about a whole family of iron-containing proteins, which define the system of cell activity and can be considered, on the one hand, as biological markers of tumor process and, on the other hand, as promising targets for anti-tumor therapy. It is known that Fe ions, like most cytotoxic agents, are capable to initiate apoptosis in cell lines of different origin. At the same time different chelators and donators of iron ions are being discovered and actively studied [21,22].
At the present stage of development of theoretical, experimental and clinical oncology considerable interest is focused on the sources of iron in the form of nanomaterials, first, as a potential delivery vector for cytostatics; second, as an exogenous modifier of endogenous iron metabolism; and third, as a factor of selective accumulation and overcoming of resistance to anti-tumor drugs (ATDR).
The aim of this study was to examine the effect of the nanocomposite (NC) and its components (magnetic fluid (MF), cisplatin (DDP)) on the level of endogenous iron exchange and the key links of genetic and epigenetic regulation of apoptotic program of sensitive and resistant MCF-7 cells.
2. Materials and Methods
The investigation was carried out in vitro on cultures of MCF-7 human breast cancer cells, sensitive (MCF-7/S) and resistant to DDP (MCF-7/DDР). The cells of original line (sensitive to ATDR) were cultivated in modified culture medium Dulbecco ISCOVE (Sigma, Germany) with adding of 10% of fetal bovine serum (Sangva, Ukraine) at the temperature of 37˚C and СО2 concentration of 5%. The cells have been reseeded twice a week with density 2 - 4 × 104 cells/cm2. We obtained a variant of this line resistant to DDP (MCF-7/DDР) by growing of cells in culture medium with adding of rising concentrations of DDP. Every two months studied cells were analyzed in order to determine the level of their resistance with 3-(4,5-dimethylthiazol-2-1)-2,5-diphenyltetrazolium bromide (MTT viability test). On the moment of investigation the level of resistance of MCF-7/DDP cells was 4.
2.1. MTT Assay
After incubation period 10 μl of MTT were added into every well of 96-well plate. Cells were incubated at 37˚C in humid atmosphere for 3 hours and then centrifuged (1500 rpm for 5 min). Violet crystals of formazan were visually detected on the bottom of wells. After removal of the supernatant 50 μl of DMSO were added into wells to dissolve formazan crystals. After 20 min of incubation at room temperature crystals totally dissolved. Optical density in wells was measured by multiwell spectrophotometer (Labsystems Multiscan PLUS, USA) at 540 nm [23].
We studied cells sensitivity and cytotoxic effect of NC and its components. NC and DDP were used in doses equal to IC20 (by DDP) for each studied cell line (sensitive and resistant). Fe3O4 concentration in medium was equal to 12 µg/ml for MCF-7/S and 48 µg/ml for MCF-7/DDP cells, respectively. After addition of these agents into cultivation medium cells were cultivated for 48 hours in standard conditions.
After cultivation we studied expression of endogenous iron metabolism proteins, apoptosis regulators, CpG-sites methylation in promoters of ferritin heavy chains and transferrin receptor 1 genes, pool of reactive oxygen species (ROS), number of cells in apoptosis and necrosis, and expression of miRNA which regulate apoptosis, cell adhesion and activity of ferritin heavy chains.
2.2. Immunocytochemistry
The cells for the immunocytochemical analysis were grown on cover glasses, incubated in fixative solution (methanol:acetone 1:1) during 2 hours at t –20˚C, than with 1% solution of bovine serum albumin (BSA) for 20 minutes. The monoclonal antibodies for defying of proteins were against transferrin receptor 1 (TFR1) (Bioworld Technology, USA), transferrin (Tf), ferritin light chains (FTL) (Abcam, USA), ferritin heavy chains (FTH) (GeneTex, USA), p53, Bcl-2 and Bax (DakoCytomation, Denmark) were applied in standard conditions of cultivation with according to algorithm of methodical approaches [24].
The estimation of results was made with the help of optical microscope (×100, oil immersion) with usage of classical method of H-Score:

where S-“H-Score” index, N1+, N2+ and N3+ -numbers of cells with low, medium or high expression of the marker [25].
2.3. Methylation-Specific PCR (MSP)
Bisulfite conversion involves the deamination of unmodified cytosine residues to uracil under the influence of hydrosulfite ion from water solution of sodium bisulfite. Such treatment doesn’t affect 5-mC and in further amplification uracils are amplified as thymines, whereas 5-mC residues get amplified as cytosines. Bisulfite conversion was performed using EZ DNA Methylation Gold-Kit (Zymo Research, USA) according to manufacturer’s protocol. Aliquots of bisulfite-modified DNA were stored at −20˚C and were used for MSP. Methylation-specific PCR was performed using the standard protocols; primer sequences are available in Table 1.
MSP-mixture contained 12.5 µl of Master Mix (Applied Biosystem, USA), 50 pM forward and reverse primers (IDT, USA), 3 ml (~50 ng) of bisulfite-converted DNA and deionized water to a final reaction volume of 25 µl. PCR reaction consisted of the following steps: denaturation of DNA at 95˚C during 10 min, 35 cycles of amplification (denaturation at 95˚C for 30 s), annealing of primers (56˚C for 20 s), polymerization (72˚C for 30 s) and final polymerization at 72˚C for 10 sec.
PCR products were analyzed by agarose gel electrophoresis in 1.2% agarose “Low EEO, Type 1-A” (“Sigma”, USA). Samples were loaded in amount of 6 ml PCRproduct in well for each sample. For electrophoresis was used Tris-acetate buffer (40 mM Tris-acetate, pH 8.0; 1 mM EDTA). After electrophoresis the results was visualized by ethidium bromide, photographed under UV light and evaluated by a computer program TotalLab v2.01.
2.4. MicroRNA Expression Analysis
Total RNA extraction from the cells was performed using a commercial set of “Rhibo-zol” (Amplisense, Russia). Concentration of isolated RNA was determined using spectrophotometer “NanoDrop 2000c” (ThermoScientific, USA). Purity of isolated RNA is controlled by E260/E280 ratio. RNA then was dissolved in TE buffer and stored at −20˚C.
For the reverse transcriptase PCR (RT-PCR) we used the reaction mixture, listed in Table 2. RT-PCR was performed “Tertsyk” thermocycler (“DNA technology”, Russia) using the following reaction parameters (Table 3). After the RT-PCR reaction product was added to a mixture of reagents (Table 4). AmpliTaq Gold Enzyme was activated and PCR was performed (40 cycles, denature-

Table 1. Primer sequences used in MSP.
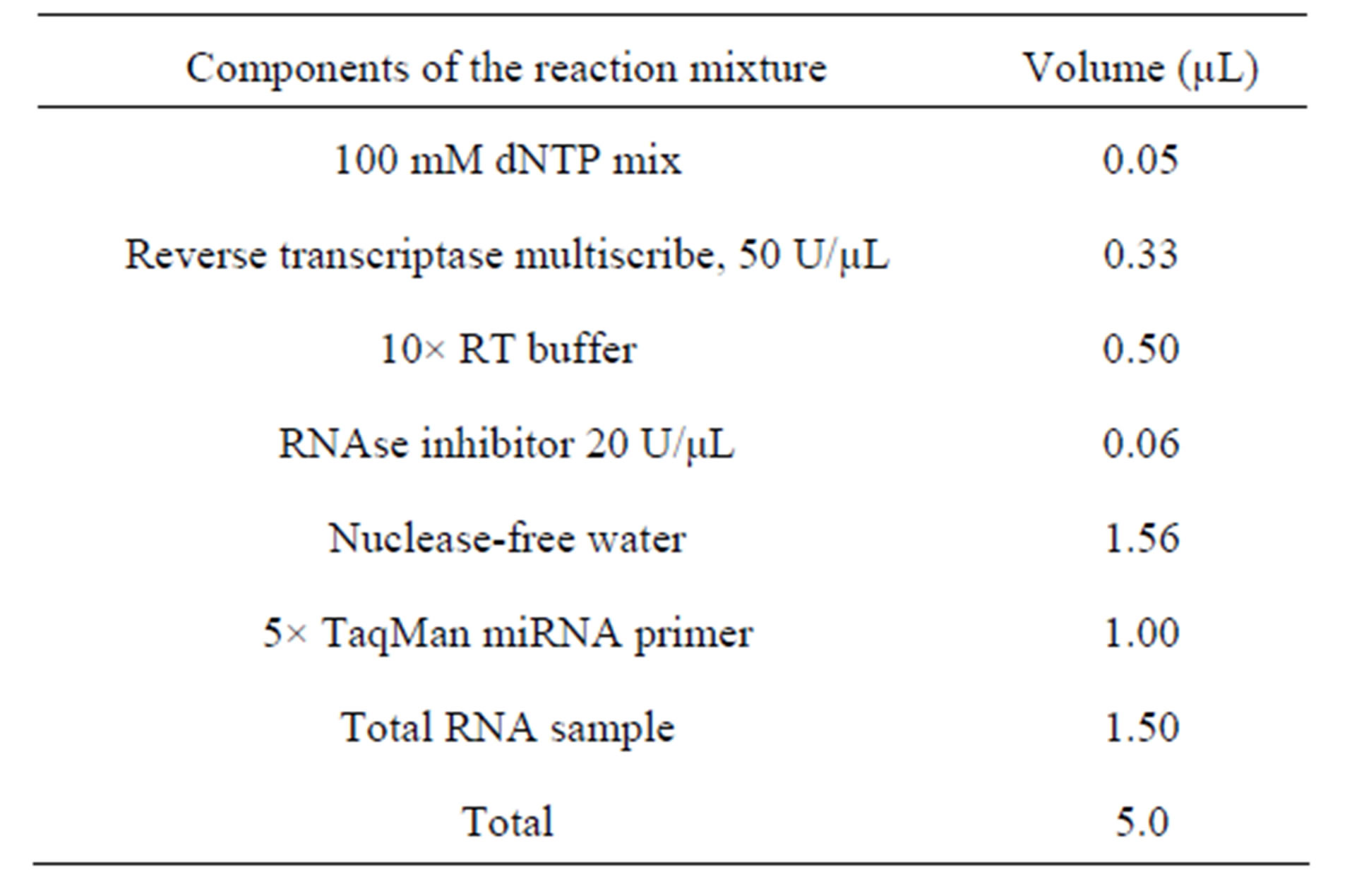
Table 2. Composition of the reaction mixture for RT-PCR.
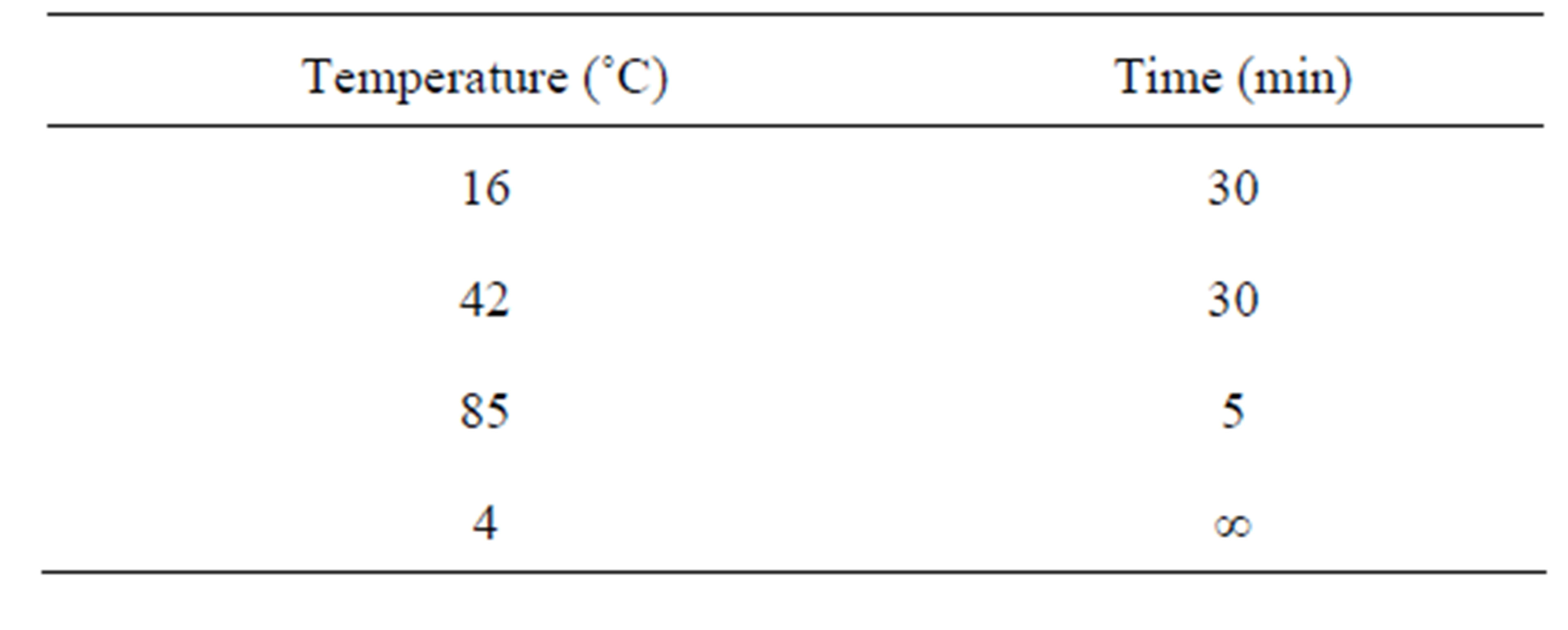
Table 3. Temperature parameters for RT-PCR.

Table 4. Composition of the reaction mixture for PCR.
tion at 95˚C for 15 sec, annealing and extension of primers at 65˚C, 60 sec).
Horizontal gel electrophoresis was performed as described above. The values of miRNA expression were normalized by calculating the miRNA/miRNA-u87 (positive control) expression ratios for each miRNA.
2.5. Measurement of Intracellular ROS
CM-H2DCFDA, a lipid soluble membrane permeable dye upon entering cells undergoes deacetylation by intracellular esterases and forms the more hydrophilic, nonfluorescent dye Dichlorodihydrofluorescein (DCFH2). This is subsequently oxidized by ROS with formation of a highly fluorescent oxidation product, Dichlorofluorescein (DCF). The generated fluorescence is directly proportional to the amount of ROS. Fluorescence was analyzed by flow cytometry. The effect of DDP, MF and NC on generation of ROS was measured in MCF-7/S and MCF-7/DDP cell lines (2.5 × 105 cells). After centrifugation (1500 rpm for 5 minutes) cells were resuspended in PBS, incubated for 30 minutes at 37˚C with CM-H2DCFDA (10 mM) for measurement of ROS. Positive control with 25 μM H2O2 was also made (data not presented). Fluorescence was acquired in the log mode and expressed as geometrical mean fluorescence channel (GMean). Acquisition was performed on 10,000 gated events.
2.6. Measurement of Annexin V Positivity
Translocation of phosphatidylserine from the inner to the outer leaflet of the plasma membrane occurs during apoptosis and can be assessed by exploiting the high binding affinity of Annexin V, a Ca2+-dependent phospholipid binding protein to phosphatidylserine. To examine whether cell death occurred via apoptosis or necrosis, propidium iodide (PI), a non-permeable stain with affinity towards nucleic acids, which selectively enters necrotic or late apoptotic cells, was used. Therefore, costaining of Annexin V and PI helps discriminate between live cells (PI and Annexin V negative), cells in early apoptosis (Annexin V positive, PI negative), cells undergoing late apoptosis (both Annexin V and PI positive) or necrotic cells (PI positive, Annexin V negative).
For detection of apoptotic cells we used apoptosis detection kit (Annexin V-FITC kit, Beckman Coulter, USA). Briefly, MCF/S and MCF/DDP (2.5 × 105/ml) were incubated with DDP, MF and NC as described above. After two washes, cells were resuspended in Annexin V binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) and Annexin V-FITC was added according to the manufacturers’ instructions. The cells were incubated for 10 minutes in the dark at 37˚C and just 5 min prior to acquisition, PI (0.1 mg/ml) was added and cells were washed and placed then in a flow cytometer.
2.7. Statistical Processing of the Results
Statistical processing of the obtained results was carried out with the help of mathematical program of medical and biological statistics STATISTIСA 6.0 and in the environment of Microsoft Excel. Calculation and comparison of the significance of differences between the average values was carried out with usage of Student’s t-criterion. Differences were considered significant with the probability not less than 95% (Р < 0.05).
3. Results
Currently it has been clearly demonstrated that the effectiveness of most ATDR depends not only on the depth of DNA violation or other cellular targets damages, but it is also associated with the apoptotic program induction, cells’ oxidative stress, impaired structural and functional state of their cytoskeleton, and changes in transport and metabolic processes, including iron metabolism. At the same time one of the biggest obstacles in the treatment of patients with malignant tumors, including breast cancer, is resistance to ATDR. According to the literature data and the results of our research, the development of resistance to cytostatic agents is accompanied by changes in the level of apoptosis regulatory-proteins, proliferation, transport, detoxication system, intercellular adhesion and receptor status [26].
At the same time, it was shown that the formation of the phenotype of drug resistance to DDP is accompanied by impaired protein metabolism of endogenous iron: increased expression of regulatory-proteins of transmembrane transport (TFR1), cell influx of iron (Tf) and ferritin (FTL, FTH) as a protein of intracellular iron storage and deposition (Figure 1, Table 5). Development of resistance to anticancer drugs also was accompanied by the shift of ferritin’s light chains from cytoplasm to cell nucleus.
It should be noted that the development of DDP-resistance in MCF-7 cells led to the increase of TFR1 expression by 200%, Tf level more than 80%, and FTL and FTH—by 50% and 75%, respectively.
Based on the data presented above, and in contrast to the searching for binding factors of Fe ions in tumor cells, we investigated the anti-tumor effect of NC containing DDP and mixture Fe2O3-Fe3O4 nanoparicles, as delivery vector for cytostatic. To elucidate the possible mechanisms of death of original and resistant cells caused by NC and its components we held a wide range of studies of molecular genetic and epigenetic markers that might be associated with iron regulatory-proteins and survival of MCF-7 and MCF-7/DDP cells.
In our previous studies with use of light and electron microscopy we showed that MF and NC were able to penetrate and accumulate in both MCF-7/S and MCF-7/DDP cells [26] using histochemical staining with potassium ferroand ferricyanide. We found that NC incorporation into MCF-7/DDP cells compared to MCF-7/S cells is much more active. Cytomorphology and electron microscopy of resistant cells with high levels of accumulated NC showed its higher cytotoxic effects in particular dystrophy, necrobiosis, necrosis and apoptosis.
It was established that cultivation of original and resistant MCF-7 cells with MF and NC was accompanied by
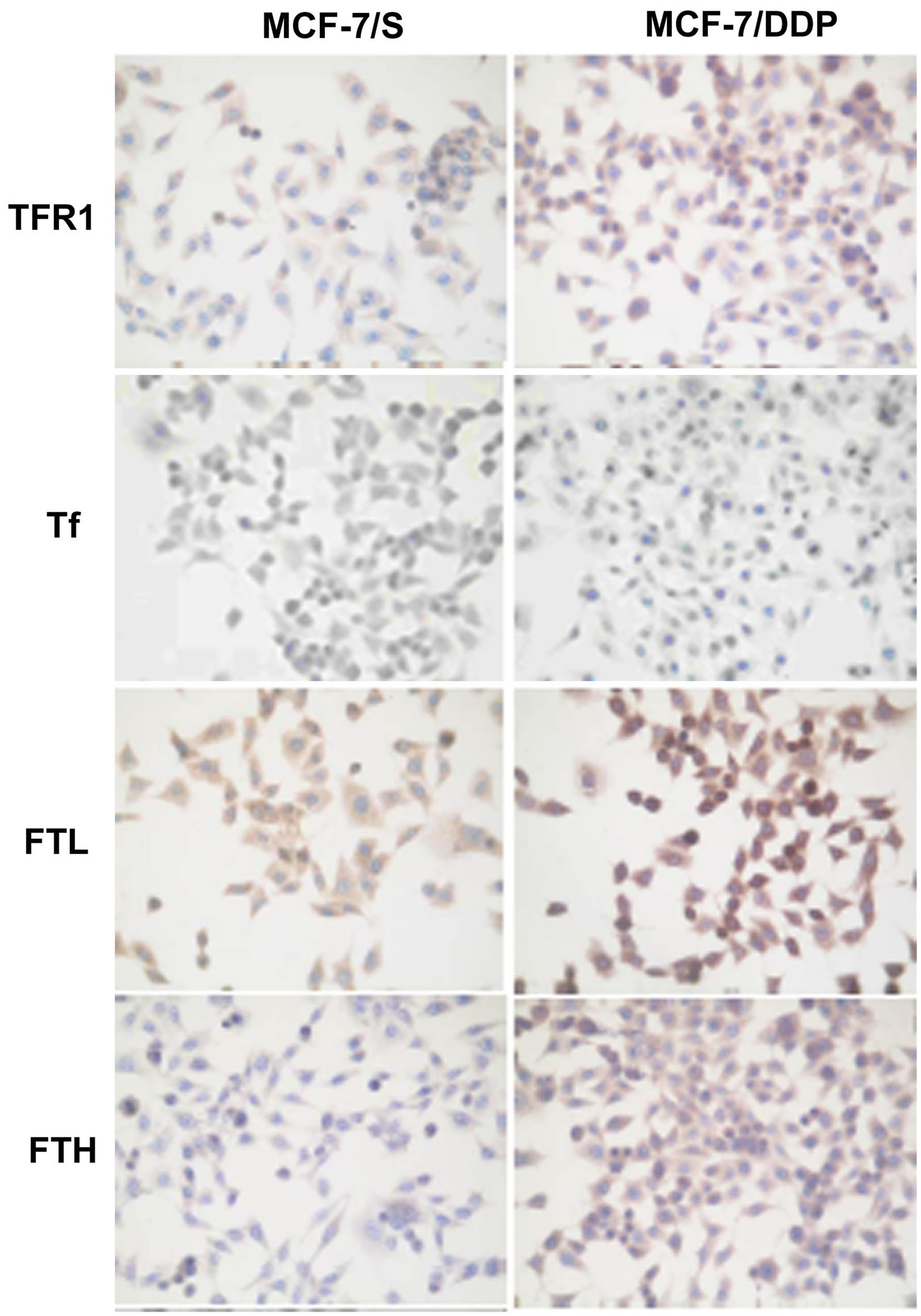
Figure 1. Expression of iron metabolism proteins in MCF-7/S and MCF-7/DDP cells.
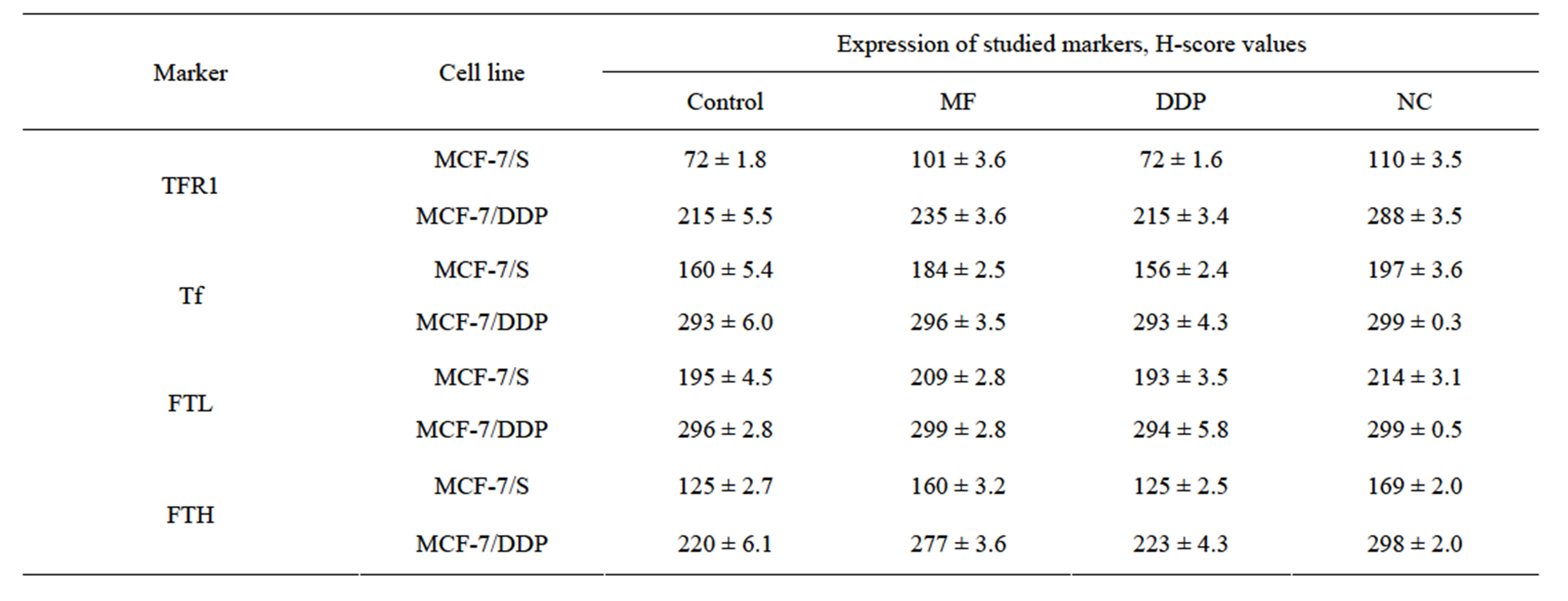
Table 5. Expression of iron-regulatory proteins in the MCF-7/S and MCF-7/DDP cells.
significant changes in the expression of the investigated iron regulatory-proteins (Table 5). Herewith, the most significant changes in original cells were observed in the levels of Tf and TFR1 expression, as well as FTL and FTH. Under the influence of MF and NC TFR1 expression level increased by 40.3% and 52.8%, and the expression level of FTH—by 28.0% and 35.2%, respectively. Significant Tf expression increase in sensitive cells was observed only under NC treatment. In resistant cells the most significant changes were found in TFR1 expression under the influence of NC and in FTH expression when MF and NC have been used (Table 5). It is noteworthy that the level of FTL expression under the influence of MF and NC did not change significantly in original and resistant cells.
At the same time we found that under the influence of NC and its components the disturbances of epigenetic regulation of some regulatory genes of endogenous iron metabolism occur. It was shown that cultivation of original and resistant MCF-7 cells with DDP, MF and NC was accompanied by hypomethylation of CpG-sites of TFR1 gene promoters and hypermethylation of CpG-sites of FTH gene promoters (Figure 2).
Free intracellular iron is known to be associated with free radicals generation. So we had investigated alterations of ROS level in parental and DDP resistant cell lines after the treatment with NC and its derivatives. It should be stressed here that ROS could induce oncogenesis in normal cells and apoptosis in cancer cells.
Significance of ROS activity isn’t definitely characterized to date. Development of DDP resistance is associated with 1.83-fold free oxygen level elevation (Table 6, Figure 3) and could be associated with failure of antioxidant system or rise of content of iron keeping proteins.
Significant increase of free oxygen radical activity was detected after NC treatment in MCF-7 cells (by 15%) and MCF-7/DDP (by 25%). Elevation of ROS level after NC treatment could be important factor on the one hand in DDP antitumor activity increase and on the other hand

Figure 2. Peculiarities of methylation CpG-sites in the genes promoters of FTH and TFR1 in original and resistant MCF-7 cells under the influence of MF, DDP and NC.


Figure 3. Flow cytometry study of ROS expression in (a) MCF-7/S and (b) MCF-7/DDP cells. Filled histogram is isotype control, ROS in control cells, ROS in cells treated with DDP, MF and NC.
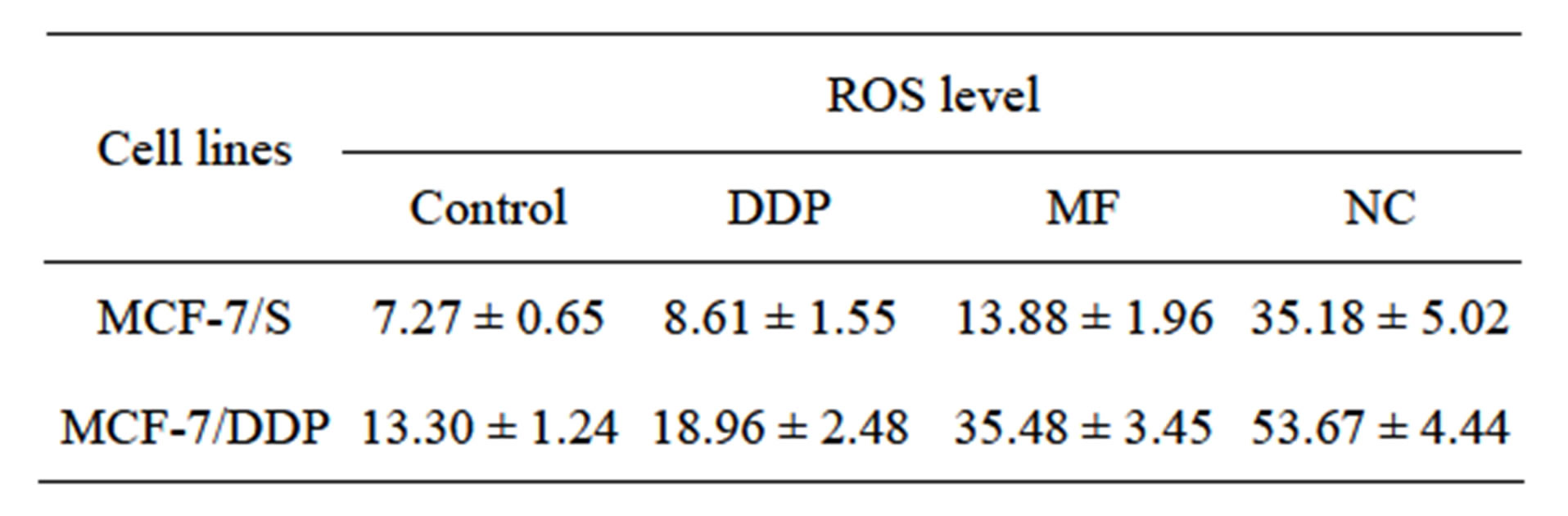
Table 6. Flow cytometry studies of intracellular ROS (GMean values).
could be a key mechanism in overcoming DDP resistance DDP is known to induce tumor cell death by the apoptotic mechanism. Meanwhile last year’s investigations suggest that NCs of different origin can also induce apoptosis in tumor cells. Thus next direction of our experiments was to elucidate the NC role in apoptosis initiation in MCF-7/S and MCF-7/DDP cells.
Table 7 and Figure 4 show that DDP induced apop-