Mineralization of Natural Spring Water in the City of Daloa (West-Central Côte d’Ivoire) ()
1. Introduction
In recent years, the issue of “drinking water for everybody” has become the subject of major international conferences and is a concern for the whole humanity. Indeed, difficulties of access to good quality water are an international problem that affects most of the world’s population, particularly in Africa. In Daloa (West-central Côte d’Ivoire), doubts about the quality of the water supplied at the tap and the low coverage rate of the drinking water supply network ( Awomon et al., 2018) are forcing part of the city’s population to turn to other sources of supply, particularly water from natural springs and traditional wells. Spring water is increasingly demanded for consumption even though studies on its quality remain very sketchy and even non-existent for some of them ( Ligban et al., 2009). However, apart from climatic and anthropogenic factors, the chemical composition of groundwater depends on the characteristics of the surrounding rocks and the reactive substances it may encounter during its infiltration or its emergence ( Boubakar, 2011). Therefore, these three factors are determining factors in the identification of the sources of groundwater mineralization ( Jain et al., 2005). It is in this context that this study which aims at determining the various processes at the origin of the mineralization of waters from natural springs of the city of Daloa. It is based on Principal Component Analysis (PCA) which is an extremely powerful tool for synthesizing information, used by several authors ( Eblin et al., 2014; Chaouki et al., 2015; Soro et al., 2019).
2. Materials and Methods
2.1. Study Area
The city of Daloa is located in the West-central part of Côte d’Ivoire, between longitudes 6˚24' and 6˚29' West and latitudes 6˚50' and 6˚55' North (Figure 1). It is the capital of the Upper Sassandra region with a population of 319,427 inhabitants
( GCPH, 2014).
The climate of the region of Daloa is a transitional humid tropical climate characterized by a dry season from November to February and a rainy season from March to October, with two maxima, one in June and the other in September (Figure 2). As a result of vast land settlement movements in the region, the forest heritage has declined considerably ( Brou, 2005). This has led to a steady decrease in local rainfall heights ( Yao et al., 2012). According to the author, the average annual rainfall in the region rose to 1212.6 mm over the period 1973-2010, with a rainfall deficit of around 16%.
2.2. Materials
2.2.1. Study Data
The data used in this study come from the results of physico-chemical analyses of water samples taken after a sampling campaign from ten (10) natural springs in the city of Daloa. They concern 14 parameters or variables: temperature (T˚C), hydrogen potential (pH), electrical conductivity (EC), redox potential (Eh), saturation rate (SR), sodium (Na+), nitrates (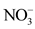 ), sulphates (
), sulphates (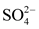 ), ammonium (
), ammonium (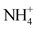 ), nitrites (
), nitrites (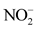 ), calcium (Ca2+), chlorides (Cl−), iron (Fe2+) and manganese (Mn2+).
), calcium (Ca2+), chlorides (Cl−), iron (Fe2+) and manganese (Mn2+).
2.2.2. Data Processing Hardware
The data processing hardware consists of the following software:
· Excel 2013 from Microsoft Office: it was used to organise and process the data;
· Statistica 7.1: It made it possible to carry out summary statistical analysis and principal component analysis (PCA) to highlight the characteristics and origin of water mineralization, respectively.
2.3. Methods
The results of the physico-chemical analyses of the natural spring water of the
![]()
Figure 2. Ombrothermal diagram of the Daloa synoptic station over the period 1990-2015.
city of Daloa have been organised and processed on Excel. Then, they were imported into the STATISTICA software to undergo statistical tests, including summary statistical analysis and finally, the normalized principal components analysis (NPCA).
2.3.1. Summary Statistical Analysis
It consists of comparing the extreme values (minimum and maximum), the central value (average) and the dispersion parameter (standard deviation) of the results of physico-chemical analyses with the drinking water guidelines for human consumption water ( WHO, 2017).
2.3.2. Normalized Principal Component Analysis (NPCA)
It is a statistical method of description which makes it possible to synthesise and classify a large number of variables in order to extract the main factors which are at the origin of the simultaneous evolution of the variables and their reciprocal relations ( Biémi, 1992). It is the subject of several applications ( Parinet et al., 2004; Mahapatra et al., 2012; Garcia et al., 2017; Zeinalzadeh & Rezaei, 2017; Zhang et al., 2020). The results are characterised by the following statistical elements: the correlation matrix, the eigenvalues of factors and the community circle. The NPCA of a study is only valid when the factorial designs studied provide more than 70% of the information sought ( Biémi, 1992). Below this limit, it is considered that the study did not take into account a large amount of useful information that remains hidden.
3. Results and Discussion
3.1. Results
3.1.1. Physico-Chemical Characteristics of Spring Water of the City of Daloa
Table 1 represents a summary statistic of the results of the physico-chemical analyses of the water from natural spring in the city of Daloa in relation to the WHO guide values.
The pH values are between 4.81 and 5.39, with an average of 5.07 ± 0.18. The spring waters of the city of Daloa are acidic. The conductivity varies between 51.7 and 225 µS/cm, with an average of 110.17 ± 53.14 µS/cm. These waters are on average very weakly mineralised with electrical conductivities lower than 100 µS/cm. Of the remaining 50%, only the water from the spring of Fatima shows average mineralization (225 µS/cm).
The average concentrations of iron (0.5 ± 1.02 mg/L) and manganese (0.61 ± 0.90 mg/L) show that these two parameters are increasing in the spring waters of the city of Daloa.
3.1.2. Correlation between Physico-Chemical Parameters
There are strong correlations between some physico-chemical parameters (Table 2). Correlations are highly significant between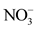 ,
, 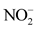 and EC (r > 0.90). A significant positive correlation was observed between Mn2+ and
and EC (r > 0.90). A significant positive correlation was observed between Mn2+ and 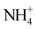 (r = 0.94). There is a strong negative correlation between Ca2+ and
(r = 0.94). There is a strong negative correlation between Ca2+ and 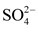 (r = −0.95) and, between Eh and pH (r = −0.81). There are mean correlations between Fe2+, Ca2+ and T˚C (r = 0.60); between Cl−, Ca2+ and Na+ (r = 0.61).
(r = −0.95) and, between Eh and pH (r = −0.81). There are mean correlations between Fe2+, Ca2+ and T˚C (r = 0.60); between Cl−, Ca2+ and Na+ (r = 0.61).
![]()
Table 1. Summary statistics of the physico-chemical parameters of spring waters.
![]()
Table 2. Correlation coefficient matrix.
3.1.3. Eigenvalues of Factors
Table 3 presents the first three factors with their eigenvalue and the variance expressed.
The percentage of variances expressed is 30.38% for factor 1, 26% for factor 2 and 14.95% for factor 3, i.e. 71.33%. The first three factors account for more than 71% of the variance expressed, and therefore of the information sought. Thus, the mechanisms that control the chemical evolution of waters from natural springs of the city of Daloa are contained in these three factors (F1, F2 and F3).
3.1.4. Variable Space Analysis
The variable space analysis concerns the factor pairs F1 × F2 and F1 × F3.
Analysis of the variable space of the factorial plane F1 × F2 (Figure 3)
The factor F1 expresses 30.38% of the variance and is determined by the following parameters: EC, 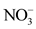 ,
, 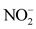 , SR and Eh. The measurement of electrical conductivity (EC) makes it possible to quickly and accurately evaluate the overall mineralization of the water. Factor 1 is therefore considered to be the axis of
, SR and Eh. The measurement of electrical conductivity (EC) makes it possible to quickly and accurately evaluate the overall mineralization of the water. Factor 1 is therefore considered to be the axis of
![]()
Table 3. Eigenvalues and percentage variance expressed.
![]()
Figure 3. Variable space of the factorial plane F1 × F2.
overall water mineralization. However, the grouping of nitrates (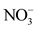 ) and nitrates (
) and nitrates (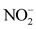 ) with electrical conductivity (EC) reflects a mineralization linked to surface inputs of elements (anthropogenic pollution) in spring water. Moreover, the highly significant correlation between
) with electrical conductivity (EC) reflects a mineralization linked to surface inputs of elements (anthropogenic pollution) in spring water. Moreover, the highly significant correlation between![]() ,
, ![]() and EC (r > 0.90) shows that the acquisition of mineralization is strongly influenced by anthropogenic pollution. In addition, the redox potential (Eh) and the dissolved oxygen saturation rate (SL) show that the presence of nitrate (
and EC (r > 0.90) shows that the acquisition of mineralization is strongly influenced by anthropogenic pollution. In addition, the redox potential (Eh) and the dissolved oxygen saturation rate (SL) show that the presence of nitrate (![]() ) and nitrite (
) and nitrite (![]() ) ions is attributed to the oxidation-reduction phenomenon. Factor 1 therefore represents the axis of mineralization of spring water linked mainly to surface inputs of elements (anthropogenic pollution) and the oxidation-reduction phenomenon.
) ions is attributed to the oxidation-reduction phenomenon. Factor 1 therefore represents the axis of mineralization of spring water linked mainly to surface inputs of elements (anthropogenic pollution) and the oxidation-reduction phenomenon.
Factor 2 expresses 26% of the information sought and is determined by the following variables: T˚C, Fe2+, Na+, Cl−. Factor 2 is an axis of mineralization linked to the water-rock contact (mineralization-residence time). This phenomenon is very weakly expressed in the acquisition of the mineralization.
Analysis of the variable space of the factorial plane F1 × F3 (Figure 4)
The factorial plane F1 × F3 represents 45.33% of the variance expressed and confirms the phenomena at the origin of the spring water mineralization observed after analysis of the space of variables of the factorial plane F1 × F2: mineralization linked to the surface input of elements (anthropogenic pollution), to the phenomenon of oxidation-reduction at water-rock contact (mineralization-residence time), very weakly expressed.
![]()
Figure 4. Variable space of the factorial plane F1 × F3.
3.2. Discussion
The study of the mode of acquisition of the mineralization of the water from natural springs in the city of Daloa was made from 14 physico-chemical parameters. The projection of these parameters in the variable space of the factorial plane F1 × F2 gave the grouping of the following parameters: electrical conductivity (EC), nitrates (![]() ), nitrites (
), nitrites (![]() ), redox potential (Eh) and dissolved oxygen saturation rate (SR) around the factor F1. The presence of
), redox potential (Eh) and dissolved oxygen saturation rate (SR) around the factor F1. The presence of ![]() and
and ![]() shows that the mineralisation of the spring water in the city of Daloa is linked both to the superficial inputs of elements (anthropogenic pollution) and to the oxidation-reduction phenomenon. This could be explained by the fact that these waters come from shallow aquifers. In fact, spring water is the natural emergence of groundwater that appears in a localised or diffuse manner on the surface of the ground. They are natural drainage areas or point outlets for ground water and can also be captured by wells or boreholes ( De Marsily & Besbes, 2017). Thus, due to their piezometric level close to the soil surface, these waters are exposed to anthropogenic pollution. The upward trend in nitrate concentrations in most groundwater has been noted by many authors ( Graveline et al., 2009; Ahoussi et al., 2013; Eblin et al., 2019). The impact of anthropogenic activities on the quality of groundwater is a major global problem and is the subject of several studies ( Bricha et al., 2007; Shahbazi & Esmaeili-sari, 2009).
shows that the mineralisation of the spring water in the city of Daloa is linked both to the superficial inputs of elements (anthropogenic pollution) and to the oxidation-reduction phenomenon. This could be explained by the fact that these waters come from shallow aquifers. In fact, spring water is the natural emergence of groundwater that appears in a localised or diffuse manner on the surface of the ground. They are natural drainage areas or point outlets for ground water and can also be captured by wells or boreholes ( De Marsily & Besbes, 2017). Thus, due to their piezometric level close to the soil surface, these waters are exposed to anthropogenic pollution. The upward trend in nitrate concentrations in most groundwater has been noted by many authors ( Graveline et al., 2009; Ahoussi et al., 2013; Eblin et al., 2019). The impact of anthropogenic activities on the quality of groundwater is a major global problem and is the subject of several studies ( Bricha et al., 2007; Shahbazi & Esmaeili-sari, 2009).
According to Ligban et al. (2009), the chemical signature of spring water, marked by low conductivities, is clearly similar to that of well water that collects the altered water table, and differs from that of borehole water that intercepts the fissured base. Analyses have shown that more than half (50%) of the spring waters studied have electrical conductivities below 100 µS/cm. The majority of these waters are very weakly mineralised. Other studies have concluded that the acquisition of groundwater mineralization is due to surface water inflow by infiltration ( Alhassane et al., 2019). Moreover, the strong correlations between nitrates (![]() ), nitrites (
), nitrites (![]() ) and EC (r > 0.90) show a strong influence of this phenomenon in the acquisition of mineralisation. On the other hand, the very weak and sometimes negative correlations between some parameters of the overall water mineralization (Ca2+, Na+, Cl−,
) and EC (r > 0.90) show a strong influence of this phenomenon in the acquisition of mineralisation. On the other hand, the very weak and sometimes negative correlations between some parameters of the overall water mineralization (Ca2+, Na+, Cl−, ![]() , etc.) and the electrical conductivity (EC) show a very weak influence of the mineralization-residence time (water-rock contact) in the acquisition mechanism of the mineralization. The low electrical conductivity values reveal that the water-rock contact time is not sufficient to allow the water to dissolve the rock and bring the mineral elements into solution. This corroborates the results of Ligban et al. (2009) which showed by calculation of saturation indices in relation to dolomite and calcite, and the use of the korjinski diagram that the low mineralization of the water from wells and natural springs in the square degree of Daloa reflects a short residence time.
, etc.) and the electrical conductivity (EC) show a very weak influence of the mineralization-residence time (water-rock contact) in the acquisition mechanism of the mineralization. The low electrical conductivity values reveal that the water-rock contact time is not sufficient to allow the water to dissolve the rock and bring the mineral elements into solution. This corroborates the results of Ligban et al. (2009) which showed by calculation of saturation indices in relation to dolomite and calcite, and the use of the korjinski diagram that the low mineralization of the water from wells and natural springs in the square degree of Daloa reflects a short residence time.
4. Conclusion
The study of the mineralization of water from natural springs in the city of Daloa (West-central Côte d’Ivoire) revealed that this water is acidic and very weakly mineralized, with high iron and manganese contents. The pH values are between 4.81 and 5.39, while half of these waters have electrical conductivities below 100 µS/cm. Average iron and manganese contents are 0.5 ± 1.02 mg/L and 0.61 ± 0.90 mg/L respectively. The Normalized Principal Component Analysis (NPCA) shows that mineralization is governed by three phenomena: surface inputs of elements (anthropogenic pollution), the oxidation-reduction phenomenon and, the water-rock contact (mineralization-residence time). The highly significant correlation (r > 0.90) between nitrates (![]() ), nitrites (
), nitrites (![]() ) and electrical conductivity (EC) reveals a strong influence of the superficial inputs of elements in the acquisition mechanism of the mineralization of these waters. It is therefore advisable to deepen research by monitoring the parameters of mineral and organic pollution in order to prevent a health risk linked to the consumption of this water.
) and electrical conductivity (EC) reveals a strong influence of the superficial inputs of elements in the acquisition mechanism of the mineralization of these waters. It is therefore advisable to deepen research by monitoring the parameters of mineral and organic pollution in order to prevent a health risk linked to the consumption of this water.