Comparative Analysis of Composition and Biodiversity of Saltlicks Forest and Control Forests, TICOYA Resguardo, Tikuna Indigenous Community, San Martin de Amayacu Sector (Colombia Amazonian Trapeze) ()
1. Introduction
The International Union for the Nature Conservation (IUCN, 2008; GAIA, 2007) defines the term sacred natural site (SNS) as: areas of land or water that have special spiritual significance for people and communities. For many indigenous people, sacred natural sites are areas where nature connects directly with the universe as a whole and the collective or individual memory of humanity merge in significant ways. Sacred natural sites can be the abode of deities, natural spirits and ancestors. The SNS are important spaces for identity and reference of a clan, tribe or community (Zapata, 2007; Von Hildebrand, 2013; Rodriguez, 2013; Rodriguez & Van der Hammen, 2011; ACAIPI, 2011; Davis, 2016) .
According to the IUCN (2008) , “one of the most important ways of conservation based on culture has been the identification and protection of sacred natural sites (SNS), which often have a valuable biodiversity; as well SNS protects key ecosystems, spaces and heritage landscapes. Indigenous, local and traditional cultures, with their respective worldviews, created protected areas before the beginning of Yellowstone National Park, which in most part of the world was used as a model for the current legislation, policy and practices of protected areas. SNS are the oldest protected areas on the planet”. For this study, a saltlicks sample was analyzed, considered SNS for Tikuna and other indigenous communities in the Colombian Amazon region.
The saltlicks are part of the called sacred sites or sites with an owner, with name and history, which are specials (Suarez, 2018) . For the Tikuna community, a saltlick is the Maloka of the owners of the jungle who are non-human beings. The Chaman negotiates access to resources, food, the healing of diseases with these owners (Franky, 2004: p. 129; Santos, 2013; Gregorio & Verschoor, 2011) .
The salt licks are nge or feminine areas. There are named the Colpas or salt water sources, where the animals go to drink to complement their food; therefore, the saltlicks are one of the most special places in the jungle to see animals (ASOAINTAM, 2007; Goulard, 1994; Moreno et al., 1997; Gregorio & Verschoor, 2011; Cabrera, 2012; Maldonado, 2012; Lozano , 2004). See Figure 1.
A saltlick for the western culture is a biotope characterized by soils with high mineral content in the form of salts, which are directly used by the animals. The factors that influence its formation are: topography, parent material, vegetation among others factors. As a result, they have a great importance for the diet of various types of animals (especially herbivores), according to previous studies, they have identified more than 39 animals species that visit them for food as licking salt in dry seasons, to supply some deficiencies of minerals that their normal diet has, or to supply the great demand of nutrients in some stages of their life such as the reproduction (Molina et al., 2018; Cabrera, 2012; Lozano, 2004; Narvaez & Olmos, 1990) .
From landscape ecology, the saltlicks are key in the functioning of forest ecosystem, indicators of its structural, functional and natural dynamic state (UNAL, 2017) .
There are few studies that make identification between saltlicks and the forest of surrounding areas. The objective of this analysis is to identify differences or similarities in the composition and structure of saltlicks and control forests.
2. Context and Methodology
2.1. Colombia Amazon Trapeze and TICOYA Resguardo1
The Amazonas region in Colombia is political administrative divide in 6 departments with 40,494,267 Ha, the Amazonas has 1,000,000 inhabitants approximately. This region represents about 10% of the Amazon basin, covers 43% of the Colombian territory, has 80% in tropical humid forests (Bht). It has an annual precipitation between 2500 and 4000 cubic millimeters by year, 30 Degrees centigrade average temperature, relative humidity up to 80%. In a year there are two periods of rains, an intense one in the months of January and February and one of less intensity, during September and December (Maldonado, 2012; Jimenez, 2013) .
The Amazon trapeze is located in the Amazonas department (Colombia), it is bounded on the north by the Putumayo River, on the south by the Amazon River, to the east by the border line with Brazil from Tarapacá to Leticia, and to the West by the borderline with the Peru, from the Yaguas river in the Putumayo department to the Atacuari River in the Amazon. In this area the Putumayo River with its main tributary, the Cotuhé, and the Amazon River with its tributaries Uassú, Atacuari, Loretoyacu and Amacayacu stand out. The Amazon trapeze comprises the Municipality of Leticia and Puerto Nariño. There are the indigenous communities Tikuna, Cocama, Yagua, Uitoto, Muinane, Tanimuca located in several resguardos (Izquierdo, 2010) .
The Tikuna community extends from the Atacuari River between Colombia and Peru to the Jutaí River in Brazil. In Colombia, they inhabit the entire Amazon trapeze with 8000 people. They share a “unique” language, with three dialects and live mainly on the banks of the Amazon river, they practice tomb and burning horticulture (chagras), fishing, hunting and tourism activities. Its resguardos are: San Antonio de los Lagos, San Sebastián, El Vergel, Macedonia, Mocagua, San Martin de Amacayacu and Cothué-Putumayo, in the Department of Amazonas (Ministerio de Cultura, 2009; Lopez, 2005) .
TICOYA resguardo, has indigenous communities of the Tikuna, Cocama and Yagua tribes, that make up a population of 5620 people, distributed in 22 communities along the Amazon rivers and its tributaries the Atacuari, Boyahuasú, Loretoyacu and Amacayacu (ATICOYA, 2007) . This resguardo was created as a conservation area by decree No. 021 of March 13, 1990. The San Martin de Amacayacu community is formed mostly of Tikunas (600 people, according to Curaca Mamerto Gregorio Vasquez 2019) (Figure 2).
The distribution of the saltlicks into this resguardo does not have cartographic information, or documents that indicate its location and biophysical characteristics. Table 1 identifies the saltlicks in prohibited and enchanted spaces. Figure 3 and Figure 4 illustrate the approximate location of some saltlicks reported by secondary information and field work carried out in the years 2015, 2016, 2017.
![]()
Figure 3. TICOYA resguardo location in the Colombian Amazonian trapeze. Source: the authors.
![]()
Figure 4. Saltlicks near of San Martin de Amacayacu, reported by the study of Cabrera (2012) in the Amacayacu Park and by work camp (2015 and 2016). Source: the authors.
2.2. Plotting and Inventory Forest Sampling
The methodological framework allow to identify and approximate the structure, composition and richness of the forest community in 6 saltlicks and its control forest in Tikuna community, TICOYA resguado—San Martín de Amacayacu (SMA)2 village (Colombia Amazon Trapeze).
This was done in three stages: 1) Identification and analysis of relevant information about saltlicks, its flora composition and structure, which particular value was given to the information on the Tikuna and Uitoto life plans3. 2) Visit and recognition of 6 saltlicks and 6 control forest in the years 2015, 2016, 2017 with the permission and the attendance of Tikuna4 people in San Martin de Amacayacu, about the status and structure of trees and the current management of these sacred spaces. 3) Analysis of biodiversity indicators, statistical analysis and conclusions.
The visit and identification of the saltlicks and control forest was carried out with the company of local knowledgeable indigenous. With them, the approximate area was calculated to establish transects of (4 × 50 m) with a distance between each transect of at least 60 meters.
In the data forest sampling were taken 21 transects in saltlicks and 21 transects in control forests see Figure 5 and basic information in Table 2.
Trees with diameters at breast height that have more or 10 cm were characterized, species data (local name) and approximate total height were recording. See Table 3.
Trees data with their respective identified species were analyzed using the Diversity, Dominance, Equity and Richness indices, described in Aguirre Ramírez (2013) and Naidu & Kumar (2016) . Which is done in order to determine the differences between the saltlicks and control forest. The indices used were
Diversity Index of Shanon-Weaver (1949)
H: is Shannon-Weaver’s diversity index;
: is the number of individuals of each species; N: is the total number of individuals; ln: it is the natural logarithm.
![]()
Table 2. Tree-based information, Saltlicks and forest control SMA-TICOYA. Source the authors.
![]()
Table 3. Base information heights and diameters trees classification for saltlicks and control forests. Source the authors.
![]()
Figure 5. Drawing of a saltlick with transect ubication. Adapted of Narvaez and Olmos 1992. Source: the authors.
Values between 0 and 1.0 of H indicate slightly biodiverse environments, between 1.0 and 3.0 correspond to moderately biodiverse environments and, of 3.0 or onwards, environments of good biodiversity (Naidu & Kumar, 2016) .
Simpson’s dominance index7 (1949)
D: is Simpson’s dominance index;
: is the number of individuals of each species; N: is the total number of individuals.
The values for this index are between 0 and 1.0. As dominance increases, diversity decreases.
Equity index
J: is the equity index of Pielou; H: it’s the Shanon-Weaver diversity index; S: is the number of species; ln: is the natural logarithm.
This index is between 0 and 1.0, where the value of 0 represents the minimum equity and 1.0, the maximum equity
Margalef’s richness index (1968)
R: is Margalef’s richness index; S: is the number of species; N: is the total number of individuals; ln: is the natural logarithm.
If the Margalef index is less than 2.0, is low richness in the environment, if it is between 2.0 and 5.0, there is moderate richness, if it is greater than 5.0, there is great richness in the ecosystem (Naidu & Kumar, 2016).
Statistical analysis
With the use of the SPSS statistical program8, an analysis was carried out to recognize if exist significant differences between the composition and structure of the saltlicks and the control forests in SMA, 673 trees and palms were used in 143 taxonomic units at the genre and species level when the tree was fully identified.
The analysis used the univariate analysis tool with the saltlick, no-saltlick (control forest) as fixed variable and the covariate area, to analyze if the species, family, genre, strata and DAP as dependent variables have significant relationships between them.
3. Results
3.1. Forest Structure
In the saltlicks, the trees that are in more proportion in the DAP A diametric class9 were identified in Patura, Venado, Huito and Maloka, saltlicks. In the DAP B class Piedra and Aramacia saltlicks. In the control forests, the trees that are in greater proportion in the diametric class DAP A are in Cpatura, Chuito and Caramacia, and in the diametric class DAP B in Cvenado, Cmaloka and Cpiedra.. See Graph 1.
The tallest trees are concentrated in stratum H3 in all the saltlicks. Maloka saltlick presents a little dominance in stratum H4. In control forests, chuito and cpiedra concentrate trees and palms in stratum H2; cpatura, cvenado, cmaloka in stratum H3 and caramacia in stratum H4. See Graph 2.
In general DAPA diametric class has more number of trees in saltlicks. In the
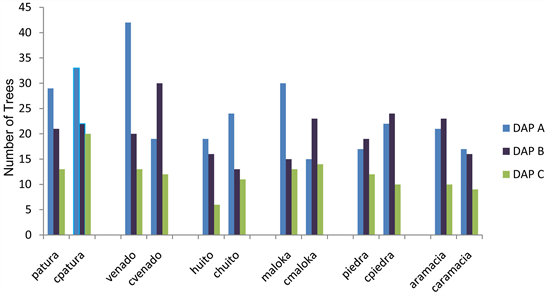
Graph 1. Distribution of the diameters under the parameters of methodology SMA. Source the authors.
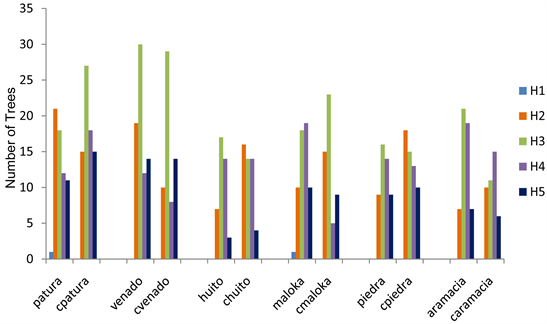
Graph 2. Distribution of the heights under the parameters methodology in SMA. Source the authors.
control forests the diameters distribution are concentrated between the DAPA and DAPB classes.
Heights distribution in saltlicks, it are concentrated in strata H2 and H3, in control forests are more trees in strata H3 and H4.
3.2. Composition
The saltlicks Patura and Venado present more number of trees. However, control forests have more number of trees in cpatura and cvenado. The number of species is more into Venado and Maloka saltlicks, and in the forest control cpatura. The number of families is higher in Venado saltlick and cvenado control forest. See Table 4 and Graph 3.
More than 25 botanical families were identified in saltlicks and control forests. The drawings Figures 6-8 show the distribution of species and families in a typical transect in 3 saltlicks and its control forests10.
The following Figure 9 present the families identified in saltlicks and control forests. The most frequent families by number of trees and palms in saltlicks are Fabaceae, Arecaceae and Myristicaceae. In control forests are Arecaceae, Fabaceae and Sapotaceae.
The consolidated distribution of the principal families of saltlicks and control forests is presented in Graph 4.
In Table 5 and Figure 10, is shown a summary of the botanical genres that have more than one tree or palm in each saltlick and control forest and that are presented in 4 or more units of analysis (saltlicks and control forest) in SMA.
The genres Astrocaryum and Virola are common in saltlicks and control forests. Nevertheless the genres Ficus and Pouteria are common in the control forests with an approximate representation of 5% and 12% respectively.
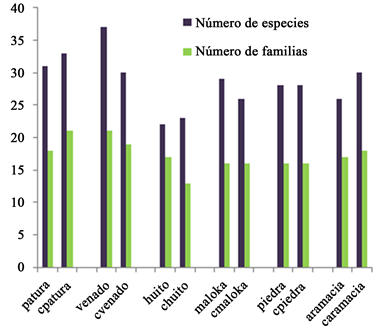
Graph 3. Distribution of species and families in saltlicks and control forests SMA. Source the authors.
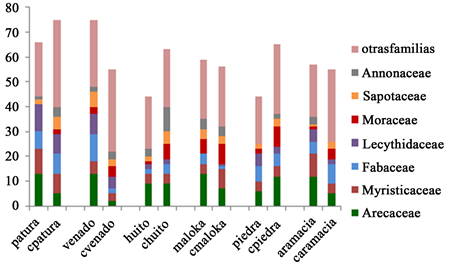
Graph 4. Distribution of families with more frequency according to the number of trees and palms identified in the saltlicks and control forests SMA resguardo TICOYA. Source the authors.
![]()
Figure 6. Left Patura saltlick drawing 12 trees and palms in 6 species, frequent families Arecaceae, Lecythidaceae, Myristicaceae. Right control forest cpatura, 15 trees and palms in 7 species, frequent families Sapotaceae, Fabaceae, Lecythidaceae source authors.
![]()
Table 4. Tree basic Information SMA, source the authors.
![]()
Figure 7. Left Venado saltlick drawing 15 trees and palms in 9 species, frequent families Arecaceae, Lecythidaceae,Fabaceae. Right control forest Cvenado, 12 trees and palms in 7 species, frequent families Sapotaceae, Annonaceae, Urticaceae source authors.
![]()
Figure 8. Left Maloka saltlick drawing 14 trees and palms in 9 species, frequent families Arecaceae, Moraceae, Myristicaceae. Right control forest Cmaloka, 13 trees and palms in 7 species, frequent families Moracea, Clusiaceae, Myristicaceae, source authors.
![]()
Table 5. Frequent botanical genres in saltlicks and forest control SMA resguardo TICOYA. Source the authors.
3.3. Statistical Analysis
With the use of the SPSS statistical program, an analysis was carried out to recognize if exist significant differences between the composition and structure of the tree in saltlicks and the control forests in SMA, 673 trees and palms were used in 143 taxonomic units at the genre and species level when the tree was fully identified.
The analysis used the univariate analysis tool with the saltlick, no-saltlick (control forest) as fixed variable and the covariate area, to analyze if the species,
family, genre, strata and DAP as dependent variables have significant relationships between them.
It can be observed that present a tendency in the forests control of trees distribution in H4 strata and in the bigger diameters. But the values are not statistically significant. See Table 6.
According to the results obtained, they have a tendency in the control forest of more species and genre, but it is not statistically significant. The variable family has more number in the saltlicks but it is not statistically significant. See Table 7.
After the significance of the families with the highest occurrence in saltlicks control forests were evaluated. The results are presented in Table 8.
The Moraceae family is representative under the statistical analysis in the control forests.
3.4. Biodiversity Indices
Table 9 shows the indices for the saltlicks and the control forests in SMA resguardo TICOYA.
Shannon-Weaver diversity index: all saltlicks and control forests have good biodiversity since their values are more or closer to 3. However Saltlicks Patura,
![]()
Table 6. Information statistical values of trees distribution by height strata and diametric category by percentage. SMA. Source the authors.
![]()
Table 7. Statistical analysis by number of trees, species, genre and families between saltlicks and control forests. Source the authors.
![]()
Table 8. Statistical analysis composition—families. Source the authors.
![]()
Table 9. Comparison between saltlicks indices and control forests SMA resguardo TICOYA. Source the authors.
Huito and Aramacia have values close to 3.0, which indicates that their environments are biodiverse but not with highest values.
The control forests cpiedra, cvenado, cpatura, cpiedra and cmaloka have a high biodiversity, since their index is more than 3.0, while caramacia and chuito are close to 3.0, which indicates that they have good biodiversity but not the highest of the sample. The forest control cpatura is the one that has the highest value in this sample indexes.
Simpson dominance index: few dominance of species can be observed in all spaces (saltlicks and control forests), the values of this index are close to zero for all inventoried forests.
Equity index of Pielou: in all inventories forests, this index is close to 1.0, which indicates that these spaces are equitable for all species.
Margalef’s richness index: all the saltlicks and control forests have good richness, because the index is higher than 5, the richest saltlicks are Venado and Maloka and the richest control forests are Cpatura and Cvenado.
According to Graph 5, the highest values of the indices are in the forest control cpatura, conversely the lowest value in the saltlick Huito. The saltlick venado and control forest cvenado have similar values of indices. The saltlick is Huito and control forest chuito have the lowest values of the data sample.
4. Discussion
For the direct observation of saltlicks and control forest in the work camp, it is important to emphasize that:
The vegetation in the saltlicks sample, (area of lickers and forest) has not been altered. The community of SMA is not allowed to down these trees or palms and
![]()
Graph 5. Distribution of the indices values evaluated in the saltlicks and control forests in SMA resguardo TICOYA. Source the authors.
it is prohibited to remove the flora or modify its vegetal coverage by chagras or other human use. Nevertheless, due the rules of management and use they have settled in these sacred spaces. For that, it is considered the flora of these 3 saltlicks untouched or with little human intervention.
The transects carried out in the control forests are outside the area of influence of the saltlicks and chagras of recent implementation. No recent trees felling or removal vegetation cover was observed. It is considered that the inventoried control forests present little anthropic alteration in their vegetation cover in a less period of 9 years.
With this background, it is notorious that in the sample of saltlicks and control forest inventoried, the number of trees with more of 10 cm of DAP is a bit more in the saltlicks than in the control forests. Otherwise is no presence of this saltlicks sample of trees with highest diameters and tallest, due to the humidity conditions and the presence of salts and other minerals in these spaces as reported by other studies. See too Bustamante et al. (2009) and De Oñate (2012).
For botanical families, the difference between the number in the saltlicks and the control forests is not important, it is observed in the distribution of trees and palms that are concentrated more than 50% in 4 families in the saltlicks. In control forests more than 50% of their trees and palms are in 6 families. The families Arecaceae, Myristicaceae, Fabaceae and Lecythidaceae are common in saltlicks and control forests12.
The number of species and genres is higher in the control forests in trees and palms with more of 10 cm of DAP, there are no dominant genre and/or species. The genres Astrocaryum sp, Iryanthera sp, Inga sp, Pouteria sp are common in the saltlicks and control forests13. Eschweilera sp and Ficus sp genres are not common, as can be seen in Table 10.
These, suggests that the composition of genres and species are different in the saltlicks and control forests. What deserves more inventories and researches in more number of samples and in all diameters of DAP for trees and palms.
5. Conclusion
Therefore, the number of trees is more in saltlicks; the number of species and of genres is more in the control forests, without being these statistically significant. It was identified more than 25 botanical families, the frequent families by more number of trees and palms in the saltlicks are Arecaceae, Myristicaceae and Lecythidaceae. In the control forest, the families with more number of trees and palms are Arecaceae, Fabaceae and Sapotaceae.
Saltlicks and control Forests in the inventoried sample, present a little different composition in families. That needs more studies to have detailed information of the composition and structure of these forest communities in other saltlicks into TICOYA resguardo.
The family Arecaceae is the one that predominates in the 6 saltlicks sample and in the control forests without being dominant.
Diversity is higher in saltlicks and control forests; their value is closer to 3. This index is highest in Venado saltlick vs forest control; in the remaining 5 saltlicks this index is a little higher in the control forest. The highest value of this index is in Cpatura.
In inventoried forests there is little dominance of species and present equity index is positive.
There is more species richness in the control forests. Venado saltlick has the richness value higher than control forest; this saltlick is the one that has the highest index of the 6 saltlicks; the forest control cpatura is the one that presents the highest index of all values.
![]()
Table 10. Genres and families that represent more than 50% of trees and palms of saltlicks and control forest inventory SMA. Source the authors.
The diametric class DAP A presents a more number of trees and palms in the saltlicks; moreover in control forests the distribution in the diameters is concentrated between the classes DAP A and DAP B. In the distribution by heights in saltlicks, it concentrates in strata H2 and H3; in control forests there are more trees in strata H3 and H4.
Funded
This work is funded by FEDER funds through the Operational Programme for Competitiveness Factors—COMPETE and by National Funds through FCT—Foundation for Science and Technology under the UID/BIA/50027/2013 and POCI-01-0145-FEDER-006821.
Annex 1
Drawings with the distribution of genres and families in a typical transect in Huito, Aramacia and Piedra saltlicks and their control forests.
![]()
Figure A1. Left Huito saltlick drawing 14 trees and palms in 8 species, frequent families Arecaceae, Myristicaceae and Annonaceae. Right control forest Chuito 16 trees and palms in 7 species, frequent families Fabaceae, Annonaceae and Arecaceae, source the authors.
![]()
Figure A2. left Aramacia saltlick drawing 18 trees and palms in 8 species, frequent families Arecaceae, Myristicaceae and lecythidadeae. Right control forest Caramacia 14 trees and palms in 7 species, frequent families Meliaceae, Sapotaceae, Myristicaceae, source the authors.
![]()
Figure A3. Left Piedra saltlick drawing 12 trees and palms in 7 species, frequent families Arecaceae, Fabaceae and Lecythidadeae. Right control forest Cpiedra 14 trees and palms in 9 species, frequent families Lauraceae, Fabaceae and Euphorbiaceae, source the authors.
Annex 2
![]()
![]()
Table A1. Tree and palm species identified in saltlicks SMA.
NOTES
1Legal and sociopolitical institution of a special nature, made up of one or more indigenous communities. With a collective property title enjoys the guarantees of private property, owns its territory and is governed, for the management of this and its internal life, by an autonomous organization protected by the indigenous jurisdiction and its own normative system (Article 21, Decreto 2164 de 1995 ).
2Legal In the text SMA is San Martin de Amacayacu.
3 ACITAM (2008) ; ACAIPI (2011) ; ASOAINTAM (2007) ; ATICOYA (2007) ; AZCAITA (2008) ; Monje Carvajal (2014) .
4Humberto Gregorio, a knowledgeable guide and recognizer of flora and fauna, Antonio Gregorio guide and translator, Robinson Gregorio guide, translator and recognizer of flora.
5Control forests name = Cpatura, CVenado, Chuito, Cmaloka, Cpiedra , Caramacia.
6Trees means in this article = trees and palms.
7Dominance occurs when one or several species (up to 3) have the environmental control conditions that influence the associated species. Dominance can influence the diversity of species in a community because diversity does not refer only to the number of species that make it up, but also to the proportion that each of them represents.
8 IBM Corporation (2018)
9 DAP A between 10 and 30 cms; DAP B between 31 and 60 cms; DAP C higher of 61 cms. Strata H2 between 2 and 10 m; strata H3 between 10 and 20 m; strata H4 between 20 and 30 m; strata H5 higher of 30 m.
10Annex 2, shows the species identified (Table A1).
11Annex 1, shows the others drawings of 3 saltlicks and controls forests in Figures A1-A3.
12In inventories carried out in permanent parcels of one hectare in the Amacayacu national park, Arecaceae, Myristicaceae and Fabaceae families are also the most frequent in the two plots.
13In inventories carried out in permanent parcels of one hectare in the Amacayacu national park genres Astrocaryum and Inga are also the most frequent in the two plots.