An Energy Supply Chain from Large Scale Photovoltaic Power Generation from Asian Cities to End Users in Japan ()
1. Introduction
Fossil fuel reserves are limited and intensive burning of hydro-carbon based fuel sources is impacting on global climate. Renewable energy sources such as wind, solar photovoltaic (PV), solar thermal, geothermal, bio-energy are drawing attention as alternative environment-friendly energy sources [1] . However, the energy density of these renewable energy sources is low. Most of them are dependent on nature and have intermittent characteristics. Therefore, it is very important to develop proper strategies and technologies to integrate these renewable energy sources into the power system network in order to fulfill the energy demand [1] .
It was proposed that photovoltaics or wind turbine would be installed oversea where a good solar radiation and wind condition are obtained and the power generated by photovoltaics and wind turbine are converted into H2 by water electrolysis using the generated power and transported to Japan [2] [3] . If large renewable energy is produced in high potential area and transported to Japan, the energy demand would be fulfilled well. Therefore, this study proposes the energy supply chain consisting of large scale photovoltaics installed in several Asian cites which have a better solar radiation than Japan, water electrolysis for H2 production and transportation by tank truck for land as well as tanker for marine. Since gases H2 at atmospheric pressure is not suitable for transportation and storage due to low energy density, this study assumes to transport H2 produced by water electrolysis to Japan by means of liquefied H2 or organic hydride.
Due to solar energy’s intermittent nature, the typical energy storage system in associated with normal PV systems such as battery bank and hydrogen produced by water electrolysis produced by the power of PV system is well known. Since the gravimetric energy density and the possible storage time of H2 are superior to battery, capacitance and flywheel [4] [5] [6] [7] , H2 is suitable for long storage and transportation. The combination system of PV and H2 produced by water electrolyzer using renewable energy sources have been studied numerically as well as experimentally [8] - [23] . Energy and exergy analysis [8] [9] [10] , economic assessment [11] [12] [13] [14] [15] , environmental assessment [16] [17] and dynamic control procedure in a short time [18] [19] [20] were reported. Though the storage characteristics of H2 produced by water electrolisys are investigated [21] [22] , there are a few reports that investigate the transportation system of H2 produced by water electrolysis using the power generated by renewable energy sources [3] [23] , which are only discussed about CO2 emission and more investigations are necessary from the viewpoint of energy assessment. In addition, there is no study considering the H2 produced by water electrolysis using the power generated by large scale photovoltaics.
In this paper, a desktop case study has been conducted on a proposed energy supply chain. The proposed energy supply chain consists of solar panels, water electrolyzer, H2 liquefaction process (or conversion process from H2 into organic hydride), transportation by tank truck for land as well as tanker for marine and fuel cell (FC). Photovoltaic power generation of megawatt class is assumed to be installed in Kuala Lumpur, Kolkata, Beijing and Ulan Bator. The power generation characteristics of PV system assumed to be installed in four Asian cities were evaluated using meteorological data of METPV-ASIA [24] . The H2 is produced by the water elecrolyzer with power generated by PV system. The H2 is assumed to be transported after liquefaction or conversion into organic hydride. To convey the H2 energy from each city in Asia to Tokyo in Japan, tank truck and tanker are considered for land and marine transportation, respectively. This study investigates the electricity generated by PV system assumed to be installed in four Asian cities and the amount of H2 produced by water electrolysis using the electricity generated by PV system. This study also investigates the energy efficiency of the proposed energy supply chain and the amount of CO2 emission in the transportation process.
2. The Proposed Energy Supply Chains
This study proposes four energy supply chains sourced from four Asian cities to end users in Tokyo Japan, with the following assumptions:
1) Megawatt class of PV array would be installed in Asian cities where solar resources are good, i.e., Kuala Lumpur (Latitude: 3.08˚N, Longitude: 101.42˚E), Kolkata (Latitude: 22.34˚N, Longitude: 88.22˚E), Beijing (Latitude: 39.54˚N, Longitude: 116.23˚E) and Ulan Bator (Latitude: 47.55˚N, Longitude: 106.55˚E). H2 is produced by water electrolyzer using the electricity generated by PV system.
2) H2 is liquefied or converted into organic hydride as a carrier.
3) Liquefied H2 or organic hydride is transported from Asian cities to the nearest seaport by tank truck.
4) Liquefied H2 or organic hydride is transported from the sea port to Tokyo, Japan by tanker. When transporting liquefied H2, the transportation loss due to the boil-off rate of tanker is counted for estimation of amount of H2 after transportation.
5) Liquefied H2 or organic hydride is vaporized or reconverted into H2 and H2 is used for FC system to generate the power, in Japan.
In this study, the process 5 is ignored in the assessment. Figure 1 shows the world map showing the location of each city to be assessed in this study.
![]()
Figure 1. Location of the cities involved in this study.
3. Methodology of the Study
3.1. Estimation of Power Generation from the PV System
The electricity generated by PV system is calculated by using the following equation [25] :
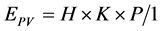 (1)
(1)
where EPV is hourly electricity generation from the PV system (kWh), H is amount of solar radiation (kWh/m2), K is power conversion factor (−), P is system’s peak capacity of PV (kWp), 1 is solar peak radiation, i.e., 1 kW/m2. The hourly solar radiation data of the reference [24] are used for calculating the hourly electric power of PV system.
In this study, the high performance PV P250a Plus produced by Panasonic whose module conversion efficiency and maximum power per module are 19.5% and 250 W [26] , respectively is adopted for PV system. The size of each PV module is 1580 mm × 812 mm × 35 mm. To calculate K, the performance value of state-of-the-art commercial devise is used. K is calculated by using the following equation [1] :
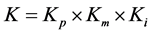 (2)
(2)
where Kp is power conversion efficiency of power conditioner (−), Km is correction factor decided by module temperature (−), Ki is power generation loss by interconnecting and dirty of module surface (−). In this study, Kp and Ki are set at 0.945 and 0.95, respectively. Kp is assumed by referring to the performance of commercial power conditioning device VBPC259B3 manufactured by Panasonic [27] . Km is calculated by the following equation [1] :
 (3)
(3)
where Tm is PV module temperature (degree Celsius), Ts is temperature under standard test condition (=25 degree Celsius) (degree Celsius), C is temperature correction factor which is 0.35 [28] (%/degree Celsius). The temperature characteristics of PV module which is adopted for this study are referred. Tm is calculated by using the following equation [29] :
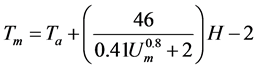 (4)
(4)
where Ta is ambient air temperature (degree Celsius), Um is wind velocity over module of PV (m/s). In this equation, the convection heat transfer by wind around the PV module is considered.
The meteorological data, such as solar radiation, the ambient air temperature, and wind velocity of the four Asian cities were from the data base of METPV- ASIA from 1999 to 2005 [24] .
Table 1 and Figure 2 show monthly and annual mean temperature [30] , and monthly precipitation [31] for the four Asian cities, respectively. These data are used for estimation of electricity generated by PV system and discussion later.
![]()
Table 1. Monthly and annual mean temperature for four Asian cities (Unit: degree Celsius).
![]()
Figure 2. Monthly precipitation for four Asian cities.
3.2. Estimation of H2 Produced by Water Electolysis
The Type-S electrolyzer manufactured by IHT [32] [33] whose H2 production rate, power consumption and electrolysis efficiency are 760 Nm3/h, 4.45 kHW/Nm3 and 79.5%, is used in this study. The amount of H2 could be produced by the power generated from PV system is calculated by the following equation [1] :
 (5)
(5)
where VH2 is amount of H2 produced (Nm3), Pe is power consumption (kWh/Nm3),  is electrolysis efficiency (−). In this study, it is assumed that the electrolyzer can be operated following the power generation characteristics of PV system every time and the produced H2 can be stored as well as used instantaneously.
is electrolysis efficiency (−). In this study, it is assumed that the electrolyzer can be operated following the power generation characteristics of PV system every time and the produced H2 can be stored as well as used instantaneously.
It is assumed that the H2 produced by electolyzer would be used to generate power through a polymer electrolyte fuel cell (PEFC) system. H2 is converted into electricity by FC following the below equation [1] :
 (6)
(6)
where  is power generation efficiency of latest PEFC stationary system based on lower heating value (=0.39) [34] , Q is lower heating value of H2 (=242) (kJ/mol). It is assumed that the energy loss for operating pump to preserve and provide gases is ignored.
is power generation efficiency of latest PEFC stationary system based on lower heating value (=0.39) [34] , Q is lower heating value of H2 (=242) (kJ/mol). It is assumed that the energy loss for operating pump to preserve and provide gases is ignored.
3.3. Evaluation of Transportation Process
It is necessary that the H2 produced by water electrolysis using the power generated by PV system is converted into a transportable media before shipped to Japan, due to low energy density of H2 in gas form. In this study, liquefaction and conversion of H2 into organic hydride are considered as the transportable media of H2.
In the case of liquefied H2 transportation, the volume of H2 is changed by 1/800 by means of liquefaction [35] . Since the energy efficiency of liquefaction of H2 is 73.6% [36] , the energy consumption of liquefaction is estimated by the energy efficiency and the amount of H2 to be liquefied. The volume of tank truck is 23 m3 [37] and the volume of tanker is 2500 m3 [38] , which are leading-edge types. The fuel for tank truck is light oil and the fuel efficiency is 2.34 km/L [39] . The fuel for tanker is heavy oil and the fuel efficiency is 0.87 km/L [40] . The speed of the tanker is 36.1 km/h [41] and the boil-off rate is 0.4%/day [38] , which is used for counting H2 loss in marine transportation. The lower heating value of light oil and heavy oil are 35.5 MJ/L [42] and 46.4 MJ/L [43] , respectively. The CO2 emission coefficient of light oil and heavy oil are 2.62 kg-CO2/L [44] and 3.41 kg-CO2/L [43] , respectively. For land transportation, it is assumed to transport from the each city to the nearest sea port, then to Tokyo port in Japan through marine transportation by the tanker. The energy consumption of transportation as well as the amount of CO2 emission in the transportation process is estimated using the distance between each city and the nearest sea port as well as the sea port and Tokyo port in Japan.
In the case of organic hydride transportation, H2 can be transported by the volume of 1/500 compared to the gases H2 at atmospheric pressure [35] . This study considers the reaction of C7H8 and H2 into C7H14 as the conversion process of organic hydride. Since the enthalpy loss of C7H14 for emitting H2, i.e., the necessary energy for emitting H2 which is an endothermic reaction is 67.5 kJ/mol-H2 [45] ; the energy consumption of conversion process of organic hydride is estimated by the enthalpy loss and the amount of H2. In this study, the energy consumption of absorbing H2 is neglected since absorbing H2 by C7H8 is an exothermic reaction. Since the organic hydride can be transported by general tank truck and tanker for petroleum oil [46] , this study assumes the volume of tank truck and tanker are 20 m3 [47] for land transportation and 20000 m3 [48] for marine transportation, respectively. The fuel for tank truck is light oil and the fuel efficiency is 2.34 km/L [39] . The fuel for tanker is heavy oil and the fuel efficiency is 0.87 km/L [40] .
4. Results and Discussion
4.1. Assessment on Characteristics of Large Scale PV System Assumed to be Installed in Four Cities
At first, the optimum tilt angle and orientation angle to install solar panel for each city is investigated using the hourly meteorological data base [24] for the four Asian cities. The monthly and annual solar radiation are estimated summing the hourly data in order to decide the annual optimum tilt angle and orientation angle for power generation of PV system. After that, the power generation characteristics of PV system under the optimum tilt angle and orientation angle condition are investigated.
As an example, Figure 3 shows the relationship between tilt angle and amount of solar radiation for different orientation angles in the case of Kuala Lumpurin January. As to orientation angle, 0 degree means south and the angle increases by 30 degree in a clockwise fashion. Table 2 lists the annual amount of solar radiation for different tilt angles and orientation angles in the case of Kuala Lumpur. From this table, it is seen that the highest amount of solar radiation is obtained at tilt angle of 20 degree and orientation angle of 270 degree (i.e. east). Table 3 lists the annual amount of solar radiation for different tilt angles and orientation angles in the case of Kolkata. From this table, it is seen that the highest amount of solar radiation is obtained at tilt angle of 30 degree and orientation
![]()
Figure 3. Relationship between tilt angle and amount of solar radiation for different orientation angles (Kuala Lumpur, January).
![]()
Table 2. Annual amount of solar radiation for different tilt angles and orientation angles in the case of Kuala Lumpur (Unit: kWh/m2).
![]()
Table 3. Annual amount of solar radiation for different tilt angles and orientation angles in the case of Kolkata (Unit: kWh/m2).
angle of 0 degree (i.e. south). Table 4 lists the annual amount of solar radiation for different tilt angles and orientation angles in the case of Beijing. From this table, it is seen that the highest amount of solar radiation is obtained at tilt angle of 50 degree and orientation angle of 0 degree (i.e. south). Table 5 lists the annual amount of solar radiation for different tilt angles and orientation angles in the case of Ulan Bator. From this table, it is seen that the highest amount of solar radiation is obtained at tilt angle of 60 degree and orientation angle of 0 degree (i.e. south).
According to Tables 2-5, the optimum tilt angel increases with increasing latitude. In addition, the optimum orientation angle is 0 degree except Kuala Lumpur. Kuala Lumpur is located near the equator and the solar altitude is the highest on the spring equinox day and the autumnal equinox day, which are different from the other cities. Therefore, the optimum orientation angle for Kuala Lumpur would be different compared to the other cities. The power of PV system is estimated under the condition installing solar panel at this optimum tilt angle and orientation angle.
Figure 4 shows the monthly electricity generated by the PV system in the four
![]()
Table 4. Annual amount of solar radiation for different tilt angles and orientation angles in the case of Beijing (Unit: kWh/m2).
![]()
Table 5. Annual amount of solar radiation for different tilt angles and orientation angles in the case of Ulan Bator (Unit: kWh/m2).
![]()
Figure 4. Monthly electricity generated by PV system for four Asian cities.
cities. In this estimation, it was assumed that the PV system consists of 10,000 solar panels (=2.5 MW; one panel = 0.25 kW). The annual electricity generated by the PV system for Kuala Lumpur, Kolkata, Beijing and Ulan Bator are 3.56 GWh, 3.74 GWh, 3.92 GWh and 4.25 GWh, respectively.
According to Figure 4, it is seen that with the same solar PV panels, the PV system in Ulan Bator could be generated the largest amount of annual power output due to its lower annual mean ambient temperature and precipitation. On the other hand, in the case of Kuala Lumpur, the annual mean temperature and annual precipitation are the highest among the four cities, thus generating the smallest annual power output. In addition, it is revealed from Figure 4 that the monthly electricity generated by the PV system is the highest in March irrespective of city.
4.2. Assessment on H2 Production by Water Electrolysis and Transportation by Liquefied H2 from Each City to Tokyo
Table 6 lists the monthly amount of produced H2 by water electrolysis using the electricity generated by PV system consisting of 10,000 PV panels or modules having total peak capacity of 2.5 MW, assumed to be installed in four cities. The annual amount of H2 produced by water elerctrolys is using the electricity generated by the PV system installed in Kuala Lumpur, Kolkata, Beijing and Ulan Bator are 28.4 Mmol, 29.8 Mmol, 31.3 Mmol and 33.9 Mmol, respectively.
Table 6 also lists the decreased volume of liquefied H2 by boil-off rate. It increases with increasing marine transportation distance and total amount of liquefied H2.
According to Table 6, the largest amount of produced H2could be delivered to Tokyo is obtained in the case of Ulan Bator, while the smallest amount is obtained in the case of Kuala Lumpur. These results follow the characteristics of electricity generated by the PV system shown in Figure 3.
Table 7 lists the annual amount of produced H2, the volume of liquefied H2,
![]()
Table 6. Monthly amount of produced H2 and delivered in Tokyo from four Asian cities.
![]()
Table 7. Transportation and conversion characteristics from four Asian cities (H2 liquefaction).
![]()
Table 8. The nearest port, land transportation distance, marine transportation distance and marine transportation days for four Asian cities.
the number of tank truck transporting for all liquefied H2, the amount of CO2 emission for all tank truck and the energy consumption for all tank truck for four Asian cities. In addition, Table 7 also lists the number of tanker transporting for all liquefied H2, the amount of CO2 emission for a tanker, the energy consumption for a tanker, the energy consumption in all transportation process, the amount of CO2 emission in all transportation process, the energy consumption for liquefaction, the electricity generated by FC in Tokyo (after transportation), the total energy consumption and the ratio of total energy consumption to calorific value of H2 delivered in Tokyo for four Asian cities. In this estimation, the 2.5 MW PV system was assumed to be installed in the source cities.
According to Table 7, it is revealed that the largest amount of CO2 emission and energy consumption for all tank truck are obtained in the case of Ulan Bator, while the smallest amount of CO2 emission and energy consumption for all tank truck are obtained in the case of Kuala Lumpur. Since the distance from Ulan Bator to the nearest seaport is very long according to Table 8 [49] and more number of tank truck is needed compared to the other cities, the amount of CO2 emission and energy consumption for all tank truck are larger in the case of Ulan Bator. On the other hand, the distance from Kuala Lumpur to the nearest seaport is the shortest and the number of tank truck is also the fewest among four cities, resulting that the amount of CO2 emission and energy consumption for all tank truck are smaller in the case of Kuala Lumpur.
As to marine transportation, it is revealed that the largest amount of CO2 emission and energy consumption for a tanker are obtained in the case of Kolkata, while the smallest amount of CO2 emission and energy consumption for a tanker are obtained in the case of Beijing and Kuala Lumpur. In this study, the amount of CO2 emission and energy consumption for a tanker depend on marine transportation distance only. Since the marine transportation distance in the case of Kolkata is the longest and those in the case of Beijing and Kuala Lumpur are the shortest among four cities, the above mentioned characteristics on marine transportation are lead.
As to all transportation process, it is revealed that the largest amount of CO2 emission and energy consumption are obtained in the case of Ulan Bator since the impact of land transportation is significant. On the other hand, it is seen that the smallest amount of CO2 emission and energy consumption are obtained in the case of Beijing. Though the impact of land transportation is not small in the case of Beijing, it is believed that this result is obtained by smaller impact of the marine transportation compared to the other cities.
It is known that the electricity generated by the FC system is about 20% of that by the PV system under the assumption of this study. Since the amount of energy is decreased by energy conversion, it is important to transport the large amount of H2 for fulfilling the energy demand of remote country.
Table 7 reveals that the total energy consumption during the transportation and the ratio of total energy consumption to calorific value of H2delivered are the largest in the case of Ulan Bator, while they are the smallest in the case of Beijing. In the case of Ulan Bator, the ratio is even greater than 1 which means it would cost more energy to transport H2 than energy (contained in H2) delivered. Table 7 implies Beijing is the best energy effective case.
4.3. Assessment on H2 Production by Water Electrolysis and Transportation by Organic Hydride from Each City to Tokyo
Table 9 lists the annual amount of produced H2, the volume of organic hydride, the number of tank truck transporting for all organic hydride, the amount of CO2 emission for all tank truck and the energy consumption for all tank truck for four Asian cities. In addition, Table 9 also lists the number of tanker transporting for all organic hydride, the amount of CO2 emission for a tanker, the energy consumption for a tanker, the energy consumption in all transportation process, the amount of CO2 emission in all transportation process, the energy consumption for liquefaction, the electricity generated by FC in Tokyo (after transportation), the total energy consumption and the ratio of total energy consumption to calorific value of H2 after transportation for four Asian cities. In this estimation, the monthly amount of produced H2 by water electrolysis using the
![]()
Table 9. Transportation and conversion characteristics for four Asian cities (organic hydride).
electricity generated by the PV system consisting of 10,000 PV panels or modules having total peak capacity of 2.5 MW as shown in Table 6 is used.
According to Table 9, it is revealed that the largest amount of CO2 emission and energy consumption for all tank truck are obtained in the case of Ulan Bator, while the smallest amount of CO2 emission and energy consumption for all tank truck are obtained in the case of Kuala Lumpur, which is the same tendency as H2 liquefaction case. Since the distance from Ulan Bator to the nearest sea port is very long according to Table 8 [49] and more number of tank truck is needed compared to the other cities, the amount of CO2 emission and energy consumption for all tank truck are larger in the case of Ulan Bator. On the other hand, the distance from Kuala Lumpur to the nearest sea port is the shortest and the number of tank truck is also the fewest among four cities, resulting that the amount of CO2 emission and energy consumption for all tank truck are smaller in the case of Kuala Lumpur.
As to marine transportation, it is revealed that the largest amount of CO2 emission and energy consumption for a tanker are obtained in the case of Kolkata, while the smallest amount of CO2 emission and energy consumption for a tanker are obtained in the case of Beijing and Kuala Lumpur, which can be explained by the same discussion as H2 liquefaction case. In this study, the amount of CO2 emission and energy consumption for a tanker depend on marine transportation distance only. Since the marine transportation distance in the case of Kolkata is the longest and that in the case of Beijing and Kuala Lumpur is the shortest among four cities, the above mentioned characteristics on marine transportation are obtained.
As to all transportation process, it is revealed that the largest amount of CO2 emission and energy consumption are obtained in the case of Ulan Bator, while it is seen that the smallest amount of CO2 emission and energy consumption are obtained in the case of Beijing, which can be explained by the same discussion as H2 liquefaction case.
After delivered in Tokyo by organic hydride, the electricity generated by FC system is about 21% of that by the PV system under the assumption of this study.
Table 9 reveals that the total energy consumption and the ratio of total energy consumption to calorific value of H2 delivered are the largest in the case of Ulan Bator, while those are the smallest in the case of Beijing, which is the same tendency as H2 liquefaction case. Since the annual amount of produced H2 in the case of Beijing is the largest compared to the other cities except Ulan Bator and the energy consumption in all transportation process is the smallest among four cities, Beijing is the best energy effective case.
Comparing the ratio of total energy consumption to calorific value of H2 delivered between H2 liquefaction case and organic hydride case, the ratio in the case of H2 liquefaction is smaller than that in the case of organic hydride. When converting gases H2 into liquefied H2 or organic hydride, the volume ratios of H2 liquefaction and conversion into organic hydride are 1/800 and 1/500, respectively. Therefore, the converted volume after H2 liquefaction is smaller than that after conversion into organic hydride. In addition, the volume of tank truck for liquefied H2 is larger than that for organic hydride, resulting that the number of tank truck for land transportation of liquefied H2 is smaller. Consequently, the ratio of total energy consumption to calorific value of H2 after transportation in the case of H2 liquefaction is smaller than that in the case of organic hydride due to impact of land transportation.
From the investigation of this study, it is revealed that the proposed energy supply chain is the optimum in the case of Beijing. On the other hand, the introduction of proposed energy supply chain is not effective in the case of Ulan Bator due to very long land transportation distance.
5. Conclusions
This study proposed an energy supply chain which consists of solar panels, water electrolyzer, H2 liquefaction process (or conversion process from H2 into organic hydride), transportation by tank truck for land as well as tanker for marine and FC. This study investigated the electricity generated by PV system assumed to be installed in four Asian cities using the meteorological data and the amount of H2 produced by water electrolysis using the electricity generated by PV system. This study also investigated the energy efficiency of the proposed energy supply chain and the amount of CO2 emission in the transportation process. As a result, the following conclusions have been drawn:
1) The largest amount of electricity generated from the same size PV systems is obtained in the case of Ulan Bator, while the smallest amount of electricity generated by the PV system is obtained in the case of Kuala Lumpur. The monthly electricity generated by the PV system is the highest in March irrespective of city investigated.
2) When assuming 2.5 MW PV panel installed in four cities in the case of H2 liquefaction, the annual electricity could be generated by the FC system in Tokyo is 0.722 GWh, 0.750 GWh, 0.807 GWh and 0.874 GWh, in the cases of Kuala Lumpur, Kolkata, Beijing and Ulan Bator, respectively. The electricity generated by the FC system in Tokyo is about 20% of the electricity generated by the PV system in the source cities. On the other hand, in the case of organic hydride, the annual electricity could be generated by the FC system in Tokyo is 0.743 GWh, 0.781 GWh, 0.820 GWh and 0.888 GWh, in the cases of Kuala Lumpur, Kolkata, Beijing and Ulan Bator, respectively. The electricity generated by the FC system is about 21% of that by PV system in source cities.
3) As expected the CO2 emission from the transportation from Ulan Batorto, Tokyo is the largest among that from four cities due to long land transportation distance.
4) Comparing the ratio of total energy consumption to calorific value of H2 delivered between H2 liquefaction case and organic hydride case, the ratio of total energy consumption to calorific value of H2 delivered in the case of H2 liquefaction is generally smaller than that in the case of organic hydride.
5) The proposed energy supply chain from Beijing to Tokyo is the optimum, while the chain from Ulan Bator to Tokyo is not energy effective at all due to very long land transportation distance.