A Novel Low Temperature Synthesis of KNN Nanoparticles by Facile Wet Chemical Method ()
1. Introduction
K0.5Na0.5NbO3 system, with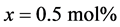 , is a solid solution of ferroelectric KNbO3 and antiferroelectric NaNbO3 and is considered as one of the most promising classes of lead-free dielectric and piezoelectric materials because of its high Curie temperature and large electromechanical coupling factor [1] .
, is a solid solution of ferroelectric KNbO3 and antiferroelectric NaNbO3 and is considered as one of the most promising classes of lead-free dielectric and piezoelectric materials because of its high Curie temperature and large electromechanical coupling factor [1] .
Unfortunately, there are some issues in the preparation of highly densified K0.5Na0.5NbO3 ceramics, like the relatively high leakage current due to the volatilization of alkaline elements during the thermal treatment and the formation of lattice defects [2] . Many attempts have been made to obtain dense KNN ceramics by using doping methods as well as different synthesis processes [3] [4] . X. Vendrell et al. reported lanthanum doped KNN thin films characterized by a lower leakage current density at low electric fields, which should be favorable for the development of low leakage current films [5] .
Usually, KNN polycrystalline powders are prepared by the conventional solid-state reaction (SSR) method using carbonates or oxides as starting materials. The particle size of these starting materials is in the micrometer or sub- micrometer range, and therefore a perovskite phase-forming minimum temperature of 850˚C is needed so that the components of the mixture have sufficient thermal energy to overcome the atomic/ionic diffusion barriers for the reaction. It should also be noted that the high-temperature heating required in this method leads not only to remarkable energy consumption and high agglomeration, but is also responsible for the large particle size of the synthesized KNN powders.
In comparison, nanocrystalline powder provides an enhanced stored energy for solid state densification due to a higher inherent surface area, ultimately resulting in a lower sintering temperature and a higher densification [6] [7] . Additionally, a lower sintering temperature is also useful to reduce the loss of highly volatile potassium content in the KNN system.
The sol-gel method has been extensively used to prepare single-phase nanocrystalline powders of piezoelectric ceramics [8] [9] . In contrast with other techniques, the sol-gel process has shown considerable advantages, including excellent chemical stoichiometry, potential of homogeneous doping, easy scale-up to mass production and lower crystallization temperature due to the mixing of liquid precursors on the molecular level [10] [11] .
Within this framework, in the present work a nanocrystalline K0.5Na0.5NbO3 ceramic was prepared by a sol-gel method involving carbonates of potassium and sodium and oxide of niobium (Nb2O5). Usually, in sol-gel method niobium alkoxides (CnH2n+1O)5Nb were used as Nb source of niobate materials, such as K(Ta,Nb)O3, LiNbO3 and similar [12] [13] . Unfortunately, their high cost and easiness to hydrolysis made them difficult to be exploited in industrial applications. Here, the cheaper and widely available Nb2O5 was changed into a water-soluble species through the chemical chelation and was employed as the Nb source to produce K0.5Na0.5NbO3 nanocrystalline system.
2. Materials and Methods
2.1. Material Synthesis
K0.5Na0.5NbO3 nanocrystalline ceramic was prepared by a sol-gel reaction technique using niobium oxide (Nb2O5, 99.9%, Sigma Aldrich chemicals, USA), potassium hydroxide (KOH, 97%, Sigma Aldrich chemicals, USA), sodium carbonate (Na2CO3, 99.8%, Sigma Aldrich chemicals, USA), potassium carbonate (K2CO3, 99%, Sigma Aldrich chemicals, USA), acetic acid (CH3COOH, 99.5%, Sigma Aldrich chemicals, USA), oxalic acid ((COOH)2∙2H2O, 99.5%, Sigma Aldrich chemicals, USA), citric acid (C6H8O7・H2O, 99.5%, Sigma Aldrich che- micals, USA) and nitric acid (HNO3, 65.0% - 68.0%, Sigma Aldrich chemicals, USA) [14] .
In this experiment, Nb2O5 (0.48 g) and KOH (1.22 g) were firstly mixed and calcined at 350˚C for 2 h to obtain a ~ 2 g of soluble potassium niobate (K3NbO4). The soluble potassium niobate (K3NbO4) was subsequently solved into distilled water (10 ml) and titrated by nitric acid (3 - 4 drops) to form a precipitate of niobium hydroxide (Nb(OH)5). After washing several times to remove potassium ion, a soluble niobium precursor was gained in virtue of the freshly precipitated Nb(OH)5 (1.69 g) chelated with oxalic acid (4.10 g), as shown in Figure 1(a). The niobium precursor solution was prepared by employing the following conditions: 2 h, room temperature, pH~3. The chemical reactions that took place can be written as follows:
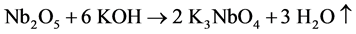
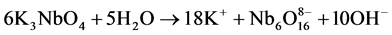
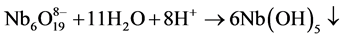
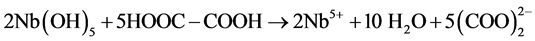
According to the chemical stoichiometry of K0.5Na0.5NbO3, the as-prepared niobium precursor solution was mixed with Na2CO3(0.96 g) and K2CO3(1.26 g) into a diluted solution of citric acid (1.92 g), and subsequently acetic acid (2-3 drops) was added to control the pH value up to 4. For obtaining a homogeneous sol, vigorous stirring for 2 h was required. Finally, the K0.5Na0.5NbO3 sol was heated at 80˚C for 12 h to prepare dried gel and the dried gels were calcined at different temperatures from 400 up to 700˚C for 4 h (see Figure 1(b)).
2.2. Characterizations
Thermo gravimetric and differential scanning calorimetry (DSC) analyses were
![]()
Figure 1. Photographs of 2Nb(OH)5 after washing with water (a) and KNN powder calcined at 600˚C (b).
performed using Netzsch TG 209 F1 TGA from room temperature up to 800˚C under dry air. The heating rate was fixed at 10˚C/min.
The crystalline structure of the sintered samples was examined using X-Ray Diffraction (XRD) analysis with  radiation in a wide range of Bragg angles
radiation in a wide range of Bragg angles 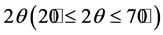 at a scanning rate of 2˚/min by Panalytical X’pert pro X-ray Diffractometer.
at a scanning rate of 2˚/min by Panalytical X’pert pro X-ray Diffractometer.
The microstructures were observed using the scanning electron microscope FESEM, Dual Beam Auriga from Carl Zeiss, at 5 keV.
FTIR spectra were obtained using Bruker, Tensor 27. Excitation was taken as the 488 nm line of Ar+ laser with 100 mW output power. The samples for FTIR analysis were prepared by KBr pellet method.
3. Results and Discussion
3.1. TGA/DSC Analysis
The sol-gel synthesis of the K0.5Na0.5NbO3 samples from alkaline carbonates and niobium oxide was followed by thermal analysis (see Figure 2).
The overall weight loss was equal to 54% from room temperature up to 800˚C. The TGA curve shows an 18% weight loss between 25˚C and 200˚C. In the same temperature range, the DSC curve shows two endothermic processes positioned at around 100˚C and 200˚C, which are associated to the departure of water molecules during the thermal decomposition of the precursor (oxalato-niobium complex) [15] . The presence of water in the carbonate-oxide powder mixture is due to the hygroscopic nature of both carbonates, particularly K2CO3, which easily absorbs a few wt% of water in normal atmosphere where the manipulation of the precursor powder takes place. The weight loss of 14% between 200˚C and 300˚C indicates an elimination of organic material.
The DSC analysis shows a corresponding endothermic peak at 300˚C. This endothermic process may be related to the release of water molecules, ammo-
![]()
Figure 2. TGA and DSC curves of K0.5Na0.5NbO3 precursor powder acquired at a heating rate of 10˚C/min.
nium ions and CO and CO2 molecules developed by the combustion of organic ligands, which led to the formation of an intermediate oxalato-niobate complex.
Between 300˚C and 400˚C, the TGA curve shows a weight loss of ~10%, which can be assigned to the elimination of CO from the mono (oxalato) complex decomposition. As a matter of fact, the thermal decomposition of simple or complex oxalates occurs with the formation of CO and CO2, which can remain in the intermediate phase, adsorbed on the surface of the solid products [16] .
Exothermic crystallization of the sodium potassium niobate phase was observed at 400˚C (Figure 2). No weight loss and no thermal effect were observed above 490˚C, indicating that no decomposition occurs above that temperature. Increased temperatures enhance the crystallinity of the calcined powder, as confirmed by the XRD analysis.
3.2. X-Ray Diffraction Analysis at Different Temperatures
Figure 3 shows the XRD patterns of the K0.5Na0.5NbO3 powders calcined at different temperatures. The sample heated at 400˚C exhibits the formation of many impurities during the initial crystallization, maybe ascribable to the hydrated carbonate phases present in an amorphous form. When temperature increases above 500˚C, a small amount of secondary phase of K3Nb6O17 was observed in XRD.
As it can be seen at 600˚C, good agreement exists between the calculated and the observed diffraction profiles. Estimated values of the unit cell lattice parameters of K0.5Na0.5NbO3 powders calcined at 600˚C were obtained using POWD software for the orthorhombic crystal structure and were found to be
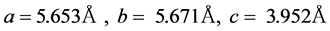 (1)
(1)
These values have been compared with , typical of a standard orthorhombic K0.5Na0.5NbO3 pattern (JCPDS, Joint Com-
, typical of a standard orthorhombic K0.5Na0.5NbO3 pattern (JCPDS, Joint Com-
![]()
Figure 3. XRD patterns of the KNN dried gels calcined at various temperatures: 400˚C, 500˚C, 600˚C and 700˚C.
mittee for Powder Diffraction file no. 32-0822). KNN average crystallite size was calculated by using Scherrer’s equation [17]
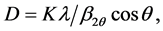 (2)
(2)
where D is the mean grain size, K = 0.94 is the Scherrer’s constant related to the shape and the index (h.k.l) of the crystal, θ is the diffraction angle, λ is the X-Ray wavelength used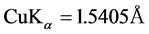 , and
, and  is the broadening of diffraction lines measured at half of their maximum intensity (in radian). The calculated mean crystallite size was estimated to be about 23.7 nm.
is the broadening of diffraction lines measured at half of their maximum intensity (in radian). The calculated mean crystallite size was estimated to be about 23.7 nm.
3.3. FESEM Investigation
Figure 4 shows the FESEM images of K0.5Na0.5NbO3 powders annealed at four different temperatures. The temperature significantly affected the microstructure of K0.5Na0.5NbO3 ceramics, as shown in the four panels of the figure. At an annealing temperature of 400˚C (Figure 4(a)), the surface structure showed inhomogeneous and abnormal grain growth and intergranular porosity was detected due to voids between the nanoparticles.
Comparatively, the microstructure of K0.5Na0.5NbO3 powder calcined at 500˚C (Figure 4(b)) was much more uniform and fine; the majority of the ultrafine particles showed a size less than 50 nm and were clustered into agglomerates, while some grains grew up to a size exceeding 200 nm. The particle size was found to be about 20 - 30 nm for the samples calcined at 600˚C (Figure 4(c)), showing a good agreement with the particle size obtained from XRD line-broadening results. By raising the temperature up to 700˚C (Figure 4(d)), the number of grains greater than 100 nm increased and the number of grains smaller than 25 nm decreased. The pores gradually disappeared as the sintering
![]()
Figure 4. FESEM micrographs of the KNN ceramics annealed at different temperatures: (a) 400˚C, (b) 500˚C, (c) 600˚C and (d) 700˚C.
temperature increased, showing the involvement of a denser microstructure. However, above 700˚C the K0.5Na0.5NbO3 nanopowder melted inhomogeneously and began to degrade.
3.4. FTIR Analysis
Figure 5 shows the FTIR spectrum of the pure K0.5Na0.5NbO3 ceramics annealed at 600˚C, in the frequency range 4000 - 400 cm−1.
In the low frequency region (below 400 cm−1), no peaks related to pure K0.5Na0.5NbO3 ceramics were highlighted. In the frequency range between 3500 and 1600 cm−1, instead, weak and broad peaks were recorded, while the broad absorbance peak of oxalic acid present in the proximity of 3300 cm−1 belongs to O?H stretching from H2O contained in oxalic acid complex. The peaks detected in the frequency range 1500 - 1200 cm−1 could arise from combinations of absorbance due to organic functional groups, i.e. asymmetric or symmetric bending of CH3 groups, but could also be related to inorganic carbonates or bicarbonates (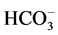 ) present in the gel [18] [19] . Since both K2CO3 and Na2CO3 spectra show a very strong absorbance in this frequency range, the origin of these peaks could also arise from the presence of an inorganic carbonate group [20] . The peak at ~1425 cm−1 showed that hydrated carbonate phases persisted in the K0.5Na0.5NbO3 nanopowder sample even after decomposition at 600˚C.
) present in the gel [18] [19] . Since both K2CO3 and Na2CO3 spectra show a very strong absorbance in this frequency range, the origin of these peaks could also arise from the presence of an inorganic carbonate group [20] . The peak at ~1425 cm−1 showed that hydrated carbonate phases persisted in the K0.5Na0.5NbO3 nanopowder sample even after decomposition at 600˚C.
It is probable that some of the hydrated carbonates detected in the FTIR spectrum are a consequence of a reaction between the K0.5Na0.5NbO3 nanopowders, after thermal decomposition, and moisture and carbon dioxide present in the air during the sample storage prior to making the FTIR analysis. It was also found that the spectrum recorded in the frequency range 800 - 500 cm−1 (see the inset of Figure 5) is not very different from those of pure KNbO3 and NaNaO3 [21] [22] .
![]()
Figure 5. Fourier transform infrared spectrum of KNN gel-powder calcined at 600˚C. The inset shows a zoom of the spectrum in the range 1200 - 400 cm−1.
3.5. Williamson-Hall Analysis
Figure 6 shows the Williamson-Hall plot of the K0.5Na0.5NbO3 nano-powder calcined at 600˚C. The Williamson-Hall analysis relies on the principle that the approximate formulae for the size broadening, ![]() , and the strain broadening,
, and the strain broadening, ![]() , vary quite differently with respect to the Bragg angle
, vary quite differently with respect to the Bragg angle![]() :
:
![]() (3)
(3)
![]() (4)
(4)
where K = 0.94 is the Scherrer’s constant, λ is the X-ray wavelength used CuKα = 1.5405Å, D is the average crystallite size, θ is the diffraction angle, C is a constant depending on the strain (typically ~4 or 5) and ε is the stored strain within alloy systems.
One contribution varies as![]() , while the other as
, while the other as![]() . If both contributions are present, their combined effects should be convoluted to get the total broadening
. If both contributions are present, their combined effects should be convoluted to get the total broadening![]() . The simplification of Williamson and Hall is to assume the convolution as a simple sum of
. The simplification of Williamson and Hall is to assume the convolution as a simple sum of ![]() and
and![]() :
:
![]() (5)
(5)
Then multiplying both sides of Equation (5) by ![]() we get:
we get:
![]() (6)
(6)
By plotting ![]() vs.
vs.![]() , as illustrated in Figure 6, and comparing the Equation (6) to the standard equation for a straight line
, as illustrated in Figure 6, and comparing the Equation (6) to the standard equation for a straight line ![]() (m = slope; c = intercept), the strain component can be obtained from the slope
(m = slope; c = intercept), the strain component can be obtained from the slope ![]() [23] . A lattice strain of about −0.32% has been determined; the negative value proves that the strain is compressive, while the low value indicates the occurrence of lattice relaxation within the K0.5Na0.5NbO3 nanocrystalline ceramic.
[23] . A lattice strain of about −0.32% has been determined; the negative value proves that the strain is compressive, while the low value indicates the occurrence of lattice relaxation within the K0.5Na0.5NbO3 nanocrystalline ceramic.
![]()
Figure 6. Williamson-Hall plot of KNN nanocrystalline powder calcined at 600˚C. The symbols are the experimental data, while the continuous blue line represents the linear fitting curve.
4. Conclusions
High-quality K0.5Na0.5NbO3 nanocrystalline powders were prepared by a facile sol-gel technique, in which Nb2O5 was changed into a water-soluble species through the chemical chelation and was employed as the Nb source. K0.5Na0.5NbO3 gel prepared using citric acid solution was found to be homogenous, thus confirming the uniform mixing of Nb5+, Na and K. Moreover, the gel precursor and the low processing temperature played a key role in the formation of the nanoparticles. The nanocrystalline K0.5Na0.5NbO3 powders prepared at a temperature as high as 400˚C exhibited well-developed crystallinity and good morphology. In addition, the mean particle size of less than 50 nm shows the potential for the production of the low-temperature sintering-densified K0.5Na0.5NbO3 bulk material. Finally, a negative and low value of the lattice strain (−0.32%) indicates the presence of a compressive strain and the occurrence of lattice relaxation in the synthesized KNN nanocrystalline ceramic. The modified low cost sol-gel method presented in this work ensured a high purity level of the fabricated KNN nanopowder and thus can be a milestone for the synthesis of other niobate solutions.
Acknowledgements
The authors acknowledge Dr. Angelica Chiodoni (CSHR, IIT, Torino, Italy) for FESEM measurements and Dr. Gian Paolo Salvador (CSHR, IIT, Torino, Italy) for FTIR and TGA/DSC measurements.