Effects of Advanced Oxidation Processes on the Decomposition Properties of Organic Compounds with Different Molecular Structures in Water ()
Received 2 June 2016; accepted 23 July 2016; published 27 July 2016

1. Introduction
Persistent organic pollutants in industrial wastewater cause serious pollution problems in the aquatic environment [1] . Some persistent organic pollutants are very toxic and are hazardous to the health of humans and other biota. Persistent organic pollutants are poorly biodegradable, meaning that natural processes cause very little decomposition of these compounds to occur, so these compounds can pollute the environment for a long time. Water is currently widely treated using activated sludge or activated carbon or using solid-liquid separation methods, but it is difficult to completely decompose persistent organic pollutants using these methods [2] - [6] . Attention has recently been paid to the use of advanced oxidation processes (AOPs) to remove persistent organic pollutants from water.
The oxidative decomposition of organic pollutants in wastewater can be achieved in an AOP by producing hydroxyl (•OH) radicals using a combination of ozone, ultraviolet light (UV), semiconductor photocatalysts, hydrogen peroxide, ultrasound, and Fenton reagents [7] . The oxidation-reduction potential of the •OH radical is 2.85 eV, which is higher than the oxidation-reduction potentials of ozone (2.07 eV), hydrogen peroxide (1.77 eV), and hypochlorous acid (1.49 eV) [8] . Therefore, •OH radicals are very reactive, and it is theoretically possible for •OH radicals to decompose almost all organic compounds to give inorganic products, such as water and carbon dioxide. •OH radicals cannot exist for very long under normal environmental conditions.
Ozone is a strong oxidizing agent that is highly selective toward specific organic compounds, such as unsaturated compounds [9] . Ozone decomposes spontaneously and then forms oxygen, so the secondary treatment of wastewater that has been treated with ozone is unnecessary, and the treated wastewater poses little risk to the environment. Additionally, UV can be used to decompose organic compounds because UV with a wavelength of less than 287 nm can cleave organic C-H bonds [10] .
A large proportion of AOP studies have been performed using ozone-hydrogen peroxide-UV processes [11] [12] , ozone-semiconductor photocatalyst processes [13] , and AOPs based on the Fenton reaction [14] - [16] . Combinations of ozone and UV have often been used to decompose organic compounds because such combinations can remove a wide range of pollutants [17] .
The decomposition of organic compounds in an AOP using ozone and UV will mainly involve oxidation by ozone and •OH radicals and cleavage by UV [18] . The mechanisms through which organic compounds are decomposed in AOPs are complicated and difficult to understand because various decomposition reactions occur simultaneously. It is thought that the efficiencies with which organic compounds are decomposed in an AOP depends on the chemical structures of the organic compounds and the AOP conditions. However, it has been reported about decomposition of various organic compounds using AOP, but there have been relatively few studies of the decomposition efficiencies achieved for compounds with different structures under different AOP conditions [19] . Therefore, we have been studied about the effects of AOPs on the decomposition properties of organic compounds with different structures.
In this study, we confirmed that the decomposition efficiencies achieved in an AOP are influenced by the AOP conditions and the chemical structures of the organic compounds being decomposed. We examined the influence of the AOP conditions on the decomposition efficiency achieved by performing tests using different AOPs (using ozone, UV, and TiO2 to generate •OH radicals) but the same organic compounds. We examined the influence of the chemical structure on the decomposition efficiency achieved by performing tests using an O3- UV-TiO2 process and different organic compounds.
2. Experimental
The experiments were carried out in a double-tube glass reactor. The inner and outer tubes had inner diameters of 22.0 and 40.0 mm, respectively, and the volume of the space between the tubes was 230 cm3. Both tubes were made of quartz glass. A photograph of the AOP reactor is shown in Figure 1. The sample solution was fed into the space between the inner and outer tubes. The volume between the point at which the ozone came into contact with the sample solution and the AOP reactor was 45.0 cm3. A low-pressure mercury lamp (UVL-10DS-33;
![]()
Figure 1. Photograph of the advanced oxidation process reactor.
SEN Lights Co., Osaka, Japan) was placed in the middle of the reactor to act as a UV source. The UV lamp had a power output of 10.5 W, and the predominant wavelengths produced were 185 and 254 nm. The air in the inner tube and the sample solution in the space between the inner and outer tubes were simultaneously irradiated by the UV lamp. Ozone has usually been generated using the silent electrical discharge method in other studies [20] , but this method consumes large amounts of electricity [21] [22] . Ozone was therefore generated in our study using the Chapman method [23] [24] , which consumed relatively little energy. Ozone was generated in the inner tube by UV irradiation, expressed by
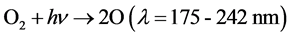 (1)
(1)
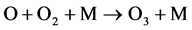 (2)
(2)
where M is the extra energy absorber which doesn’t participate in a chemical reaction directly. M is any molecule in the atmosphere, and M is usually another O2 or N2 [23] [24] . When an oxygen molecule is irradiated with UV light (wavelength 175 - 242 nm), the oxygen molecule dissociates to ground-state oxygen atoms; a ground- state oxygen atom reacts with an oxygen molecule in the presence of a third reactant such as a coexistent gas (M), and ozone is generated. The coexistent gas is not directly involved in the reaction, but it is used to absorb the excess energy generated by ozone formation [25] .
The generated ozone was transferred by an air pump (MASTER FLEX 7523-40; Cole-Parmer Instrument Co., Vernon Hills, IL, USA) through a stainless steel pipe from the inner tube to the sample solution. Ozone was produced continually because the top part of the inner tube was open to the air. Some of the ozone would have dissolved in the sample solution and been transferred to the outer tube with the sample solution. •OH radicals were generated by irradiating ozone in the aqueous solution between the inner tube and the outer tube with UV at a wavelength <310 nm [26] - [30] , expressed by
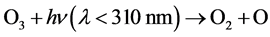 (3)
(3)
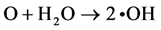 (4)
(4)
The generated •OH radicals approach each other and become surrounded by water clusters; the •OH radicals are bound immediately, by the cage effect, and then hydrogen peroxide is generated [27] - [30] .
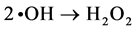 (5)
(5)
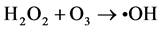 (6)
(6)
Ozone is unstable in solution, and •OH radicals are generated by an autolytic process [30] .
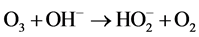 (7)
(7)
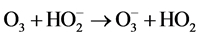 (8)
(8)
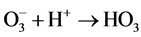 (9)
(9)
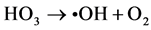 (10)
(10)
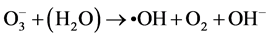 (11)
(11)
The outer tube was able to be replaced with a tube that was coated on the inside with a photocatalyst. The photocatalyst used was TiO2, which is chemically stable and relatively harmless. The TiO2 coating was applied using a dip-coating sol-gel method [31] . The TiO2 sol contained [(CH3)2CHO]4Ti, anhydrous C2H5OH, distilled water, and HCl at a [(CH3)2CHO]4Ti: anhydrous C2H5OH: distilled water: HCl molar ratio of 0.100:0.800: 0.100:0.00800. The inside of the outer tube was dip-coated with the TiO2 sol, then the tube was dried at room temperature for 10 min and then calcined at 923 K. The tube was analyzed by X-ray diffractometry, and this confirmed that the TiO2 film produced was entirely in the anatase phase. The O3-UV-TiO2 and UV-TiO2 processes were performed using the outer tube that had been coated with TiO2. Irradiating TiO2 with UV at a wavelength of <400 nm causes oxidation-reduction reactions to occur, allowing organic compounds to be decomposed.
Acetic acid, adipic acid, p-benzoquinone, catechol, formic acid, fumaric acid, glyoxylic acid, maleic acid, and oxalic acid were treated using the apparatus described above, individually. The molecular structures and chemical properties of the compounds that were tested are shown in Table 1. We found that the number of carbon
![]()
Table 1. Molecular structures and chemical properties of the test compounds.
atoms in a compound, the functional groups present, and the presence of a benzene ring affected the TOC removal efficiency that was achieved. Sample solutions containing 0.500 mmol/dm3 of acetic acid, adipic acid, p-benzoquinone, catechol, formic acid, fumaric acid, glyoxylic acid, maleic acid, or oxalic acid were prepared. Each sample solution (1000 cm3) was circulated through the system at a flow rate of 1000 cm3/min. The air containing ozone that had been generated was fed into the sample solution at a flow rate of 200 cm3/min. Samples were collected at certain times during a test. The total organic carbon (TOC) content of the solution was determined using a TOC meter (multi N/C2100S; Analytik Jena AG, Jena, Germany).
The effects achieved using five different AOPs (O3 alone and UV alone, and the combinations O3-UV, UV-TiO2, and O3-UV-TiO2) were examined. When O3 alone was used, UV light was intercepted by covering the outside of the inner tube. The O3-UV process was performed by irradiating the sample solution with UV and supplying the solution with ozone. The O3-UV-TiO2 process was performed using the outer tube that had been coated with TiO2, supplying ozone, and irradiating the system with UV. Ozone was supplied at a rate of 0.0760 mg/min (measured using the iodometric method [32] ). No air containing ozone was supplied in the UV alone and UV-TiO2 process.
3. Results and Discussion
3.1. Removal Efficiencies Using Different AOPs
The TOC removal efficiencies achieved when catechol and p-benzoquinone (both aromatic compounds) were treated using the five different AOPs are shown in Figure 2. The standard deviation was at most 2.66%. The TOC contents of the catechol and p-benzoquinone solutions decreased only a little when the solutions were treated using O3 alone, the TOC removal efficiencies being <10% in 660 min. This was because too little ozone was supplied (the ozone flow rate was 0.0760 mg/min) to decompose the catechol and p-benzoquinone effectively. The TOC removal efficiencies achieved in the catechol and p-benzoquinone tests were clearly higher when the O3-UV process was used than when UV alone was used. The TOC removal efficiencies achieved in the catechol and p-benzoquinone tests were also higher when the O3-UV-TiO2 process was used than when the UV-TiO2 process was used. The catechol TOC removal efficiency after 300 min was 34.7% higher when the O3-UV process was used than when UV alone was used, and the catechol TOC removal efficiencies obtained by the O3-UV process and UV alone for 300 min were about 35.6 times higher than for O3 alone. The p-benzoqui- none TOC removal efficiency after 300 min was 25.6% higher when the O3 UV process was used than when UV alone was used, and the p-benzoquinone TOC removal efficiencies obtained by the O3UV process and UV alone for 300 min were about 4.57 times higher than for O3 alone. We concluded that more catechol or p-benzoqui- none was removed when the O3-UV process was used than when O3 alone was used because •OH radicals, generated when the ozone was irradiated with UV [33] , would have been present during the O3-UV process. We concluded that aromatic compounds could not be effectively decomposed using O3 alone and that it took a long time to decompose aromatic compounds using UV alone. However, aromatic compounds could be more quickly decomposed using a combination of O3 and UV. The catechol TOC removal efficiency after 300 min was 34.0%
![]()
![]()
Figure 2. Removal of TOC when the aromatic compounds (a) catechol and (b) p-benzoquinone were treated.
higher using the O3-UV-TiO2 process than using the UV-TiO2 process, and the catechol TOC removal efficiencies obtained by the O3-UV-TiO2 and UV-TiO2 processes were about 34.9 times higher than for O3 alone. The p-benzoquinone TOC removal efficiency after 300 min was 41.0% higher using the O3-UV-TiO2 process than using the UV-TiO2 process, and the p-benzoquinone TOC removal efficiencies obtained by the O3-UV-TiO2 and UV-TiO2 processes were about 7.31 times higher than for O3 alone. It is possible that more •OH radicals were generated during the O3-UV-TiO2 process than during the UV-TiO2 process. In addition to the •OH radicals generated when ozone was irradiated with UV, the presence of both ozone and TiO2 would have promoted the generation of •OH radicals. Ozone adsorbed onto TiO2 captures electrons, produces ozonide ions, and generates •OH radicals [33] . Therefore, the presence of •OH radicals will strongly increase the efficiency at which aromatic compounds will be degraded in an AOP.
The TOC removal efficiencies achieved for maleic acid and oxalic acid (both open-chain compounds) using the five AOPs are shown in Figure 3. The standard deviation was at most 2.92%. The TOC removal efficiencies achieved using the different AOPs followed different trends for the open-chain compounds and the aromatic compounds. The TOC removal efficiencies for the aromatic compounds decreased in the order O3-UV-TiO2 > O3-UV > UV-TiO2 > UV alone > O3 alone. However, the TOC removal efficiencies for the open-chain compounds decreased in the order O3-UV-TiO2 ≈ UV-TiO2 > O3-UV > UV alone > O3 alone. In other words, the TOC removal efficiencies for the aromatic compounds were higher using the O3-UV process than using the UV-TiO2 process, but the TOC removal efficiencies for the open-chain compounds were higher using the UV-TiO2 process than using the O3-UV process. The TOC removal efficiencies for the open-chain compounds were about the same when the UV-TiO2 process and the O3-UV-TiO2 process were used, i.e., adding O3 to the UV-TiO2 process did not improve the TOC removal efficiencies for the open-chain compounds. It was thought that the improvement of the decomposition efficiency was not observed at O3-UV-TiO2 process compared with UV-TiO2 process because the photocatalytic activity of TiO2 was high enough to decompose the open-chain compounds. In other words, it was considered that the combination of UV and TiO2 was effective against the cleavage of C-C bonds in open-chain compounds. The UV-TiO2 process was one of the most effective methods for degrading open-chain compounds, and using the UV-TiO2 process could be cheaper than using the O3-UV-TiO2 process, because the UV-TiO2 process does not involve the use of ozone. We therefore concluded that the UV-TiO2 process was the optimum method for degrading open-chain compounds.
3.2. Removal Efficiencies for Compounds with Different Structures
3.2.1. Influence of the Number of Carbon Atoms
Adipic acid, formic acid, fumaric acid, maleic acid, and oxalic acid were decomposed using the O3-UV-TiO2 process, individually. The results are shown in Figure 4. The standard deviation was at most 2.27%. The TOC removal efficiency increased in the order adipic acid, maleic acid, fumaric acid, oxalic acid, formic acid, i.e., the TOC removal efficiency increased as the number of carbon atoms decreased. Intermediates with fewer carbon atoms would have been formed before the compound was completely decomposed to form H2O and CO2. We concluded that the fewer carbon atoms a molecule contained, the faster the molecule was converted into H2O
![]()
![]()
Figure 3. Removal the TOC when the open-chain compounds (a) maleic acid and (b) oxalic acid were treated.
and CO2 (i.e., the TOC was removed more quickly as the number of carbon atoms decreased). Lesko et al. [34] studied the kinetics of the degradation of phenol (as TOC) in an O3-ultrasound process. They reported that a plot of [TOC]/[TOC]0, where [TOC]0 is the initial TOC value, against the reaction time when phenol was degraded gave a straight line, i.e., the degradation of the TOC in the phenol tests followed pseudo-zero-order kinetics. We assumed that the degradation of the TOC when adipic acid, fumaric acid, maleic acid, oxalic acid, and formic acid were treated using the O3-UV-TiO2 process followed pseudo-zero-order reaction kinetics. Plots of our [TOC]/[TOC]0 values against the reaction time are shown in Figure 5. The pseudo-zero-order rate constants and the linear correlation coefficients (R2) for the removal of the TOC when adipic acid, fumaric acid, maleic acid, oxalic acid, and formic acid were treated are shown in Table 2. The correlation coefficients for the pseudo-zero-order rate constants that were calculated were all >0.955. These results confirmed that the removal of the TOC when organic compounds were treated using the O3-UV-TiO2 process in our tests followed pseudo- zero-order kinetics. The TOC degradation rate constants are plotted against the number of carbon atoms in the molecules in Figure 6. The curve was fitted with an exponential function, and this gave an R2 value of 0.995. These results confirmed that there was an exponential relationship (y = 0.0239e−0.366x) between the number of carbon atoms in a molecule and the TOC degradation rate constant for compounds with the same functional group.
![]()
Figure 4. Removal of the TOC when compounds containing different numbers of carbon atoms were treated.
![]()
Figure 5. Changes in the [TOC]/[TOC]0 ratio over time when compounds containing different numbers of carbon atoms were treated.
![]()
Figure 6. Relationship between the number of carbon atoms and the rate constant.
![]()
Table 2. Rate constants and R2 values for the removal of the TOC of open-chain compounds using the O3-UV-TiO2 process.
3.2.2. Influence of the Functional Group
The TOC removal efficiencies achieved for acetic acid, glyoxylic acid, and oxalic acid using the O3-UV-TiO2 process and plots of the [TOC]/[TOC]0 values against the reaction time are shown in Figure 7. The standard deviation was at most 1.98%. Acetic acid, glyoxylic acid, and oxalic acid are carboxylic acids with two carbon atoms. One of the functional groups in acetic acid, glyoxylic acid, and oxalic acid is a methyl group, an aldehyde group and a carboxyl group, respectively. The TOC degradation rate constants for acetic acid, glyoxylic acid, and oxalic acid were 0.00604, 0.00843, and 0.0115 min−1, respectively, i.e., the rate at which the TOC was removed increased in the order oxalic acid, glyoxylic acid, acetic acid. Sillanpää et al. [26] reported that glyoxylic acid was converted into oxalic acid when EDTA was decomposed using an AOP. The rate constant was lower for the degradation of glyoxylic acid than for the degradation of oxalic acid in our study, and this could have been because glyoxylic acid was oxidized to give oxalic acid. The rate constant was lower for the degradation of acetic acid than for the degradation of glyoxylic acid, and this could have been because acetic acid was oxidized to give glyoxylic acid. The possible pathway for the degradation of organic compounds with two carbon atoms during the O3-UV-TiO2 process is shown in Figure 8.
3.2.3. Influence of the Presence of a Benzene ring
The TOC removal efficiencies achieved for catechol, p-benzoquinone, and adipic acid using the O3-UV-TiO2 process are shown in Figure 9. The standard deviation was at most 1.27%. Each of these compounds contains six carbon atoms. The TOC was clearly removed more quickly in the initial stages of the reaction when adipic acid was treated than when catechol and p-benzoquinone were treated. Benzene rings were degraded less effectively than C-C bonds, and this would have been because a benzene ring will generally be more stable than a C-C bond. The [TOC]/[TOC]0 values are plotted against the reaction time in Figure 10. Two different rate constants were found for the removal of the TOC when aromatic compounds were treated. The rate constant for catechol was 0.00127 min−1 for the first 180 min, and the degradation process continued to follow pseudo-zero- order kinetics but at a faster rate (k = 0.00376 min−1) after 180 min. The results for p-benzoquinone were similar
![]()
Figure 7. Removal of the TOC and changes in the [TOC]/[TOC]0 ratio over time when compounds with different functional groups were treated.
![]()
Figure 8. Degradation pathway for an organic compound containing two carbon atoms during the O3-UV-TiO2 process.
![]()
Figure 9. Removal of the TOC when aromatic compounds (catechol and p-benzoquinone) and an open-chain compound (adipic acid) were treated.
to the results for catechol, the p-benzoquinone rate constant being 0.000927 min−1 for the first 145 min, then 0.00412 min−1 (still following pseudo-zero-order kinetics). It has previously been reported that open-chain compounds are generated when aromatic compounds decompose [34] - [36] . It could therefore be concluded that benzene rings were cleaved to generate open-chain compounds in the first stage of the degradation process, and then that the open-chain compounds generated decomposed in the second stage. It is more difficult to cleave a benzene ring than a C-C bond, and the TOC concentration in the sample solution changed only a little in the first stage of the degradation process. This explained why the rate constant was lower for the first stage than for the second stage of the degradation process.
We believe that almost all of the benzene rings had been cleaved by the end of the first stage of the degradation process. The rate constants for the second stage were between the rate constants for adipic acid (0.00280 min−1) and maleic acid (0.00516 min−1). We assume that adipic acid and maleic acid were generated in the proportions x% and y%, respectively, by the end of the first stage. The rate constants for catechol, adipic acid, and maleic acid were used to calculate x1 and y1 values for catechol, and the x1 and y1 values that were found were
![]()
![]()
Figure 10. Changes in the [TOC]/[TOC]0 ratio over time when catechol, p-benzoquinone, and adipic acid were treated using the O3-UV-TiO2 process.
59.3 and 40.7, respectively. These values indicated that 59.3% adipic acid and 40.7% maleic acid had been generated by the end of the initial stage of the catechol degradation process. Treating the p-benzoquinone results in the same way indicated that 44.1% adipic acid and 55.9% maleic acid had been generated by the end of the first stage of the p-benzoquinone degradation process.
4. Conclusions
The effects of AOPs on the decomposition properties of organic compounds with different chemical structures were confirmed in this study.
It is necessary to use an O3-UV-TiO2 process to decompose compounds containing benzene rings as quickly as possible. However, the TOC was removed at a similar rate when open-chain compounds were treated using the O3-UV-TiO2 and UV-TiO2 processes. We therefore conclude that the UV-TiO2 process degraded the open-chain compounds most effectively, and that the O3-UV-TiO2 process did not need to be used to decompose open-chain compounds.
The TOC was removed more slowly when aromatic compounds were treated than when open-chain compounds were treated. The TOC removal efficiency increased as the number of carbon atoms in the molecule being treated decreased. Organic compounds containing carboxyl groups were more easily decomposed than compounds containing aldehyde groups, and compounds containing aldehyde groups were more easily decomposed than compounds containing methyl groups. The removal of the TOC when organic compounds were treated using the O3-UV-TiO2 process followed pseudo-zero-order kinetics.
Acknowledgements
This research was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) Supported Program for the Strategic Research at Private Universities, 2012-2016, and by a Kansai University grant-in-aid for the promotion and improvement of education and research, 2014, “Development of inorganic membranes and membrane reactor using inorganic membranes”.
NOTES
![]()
*Corresponding author.