Anticorrosive Effect of Electrochemical Manganese Dioxide By-Products in Reinforced Concrete ()
1. Introduction
The reinforced concrete is the most widely used building material on the world because of high mechanical properties and durability against corrosion. However, the reinforced concrete allows entry of corrosive elements in the mass, thus reducing the life of the constructions. The high porosity and the compressive strength of concrete are related with the type of aggregate which are used and the ratio cement:water [1] .
The corrosion of steel in concrete is an electrochemical process which involves the transfer of electrons from one area to another. For corrosion to occur four basic elements are required: Anode is the site where corrosion occurs from which current flows; cathode is the site where corrosion does not occur but to which corrosion current flows; electrolyte is the pore solution in concrete, metallic path which allows current returns and circuit [2] .
In normal concrete the embedded steel is protected by the passive oxide layer [3] , which is often formed in the high alkaline environment on the steel surface such as concrete pore solution of which the pH ranges from 12.5 to 13.5 [4] . However, the passivation layer in concrete can be removed by the chemical reaction between the oxide layer and externally intruded chloride ions [5] which decrease the pH value [6] and the present of carbon dioxide and other acidic gases in the atmosphere can dissolve in the pore solution of concrete and form carbonic acid [7] . The reaction occurs mainly with calcium hydroxide and produces calcium carbonate. The above reaction severely reduces the pH to as low as 8 and as a result the passivation oxide film is broken out.
The chloride threshold level (CTL) depends from total and free chloride ions and the ratio of [Cl−]:[OH−] [8] [9] . The corrosion products over the reinforcement steel due to the larger volume than steel causing tensile stresses with result in cracking of the concrete [10] . Many investigators [11] - [16] have reported the protection of reinforced concrete. The most widely protective methods of reinforced concrete are cathodic protection, organic coatings and mineral additives such as pozzolans (volcanic ash, fly ash), blast furnace slag, silica fume and others. On the other hand, the use of organic and inorganic industrial wastes in concrete contributes to protecting the environment and reducing the cost of production of cement.
The main objective of this study is the theoretical and experimental investigation of the use of EMDW as a replacement of cement for development composite cement structures, resistant in rich chloride environment. Moreover, the used by-product in cement industry helps to reduce CO2 emissions and to protect the environment.
2. Experimental Procedure
2.1. Production of Electrochemical Manganese Dioxide & By-Products
The most thermodynamically stable form of manganese dioxide is β-MnO2 (pyrolusite), which consists of corner sharing octahedra in the a-b plane, and edge sharing octahedra in the c direction [17] . Ramsdellite has an orthorhombic unit cell and the structure. The γ-MnO2 structure is best described as a random microscopic intergrowth between the pyrolusite and ramsdellite forms of manganese dioxide.
The production of Electrochemical Manganese dioxide (EMD) initially includes milling of manganese ore and limestone. After that, leaching with SO2 to form a manganese sulfate solution. The next stage is the removal impurities, potassium, iron and heavy metals from the solution and finally EMD is plated onto titanium anodes and packaged as a fine powder. EMD wastes (EMDW) produced in two stages of filtration/leaching and includes mainly calcium sulphate, ferrous sulphate and heavy metals (Table 3). Electrolytic Manganese Dioxide (EMD) is used as a cathode mixture material for dry-cell batteries, such as alkaline batteries, zinc-carbon batteries, rechargeable alkaline batteries etc. [18] . In Greece EMD produced from TOSOH HELLAS A.I.C. The production process is shown in Figure 1.
2.2. Materials and Preparation of Mortar Specimens
Cylindrical reinforcement mortar specimens in diameter 50 mm and height 100 mm were prepared. Steel rebars type B500C Tempcore [21] were used with diameter 10 mm and 100 mm length. The chemical composition of reinforcement steel is given in Table 1 while the Table 2 presents the chemical analysis of EMD and EMDW.
As is known, the pozzolanic properties of materials related to the existence silicon oxide and its percentage in the additive (EMDW) is insignificant (Table 2). On the basis of chemical composition, the replacement of aggregates (sand) would be more right. However, the choice of specific material has been made because of the fact that EMDW is more fine than cement and likely to improve the mechanical properties of concrete. Moreover, the waste of EMD production process contains heavy metals which are harmful for the environment and the deposition of material costs. The EMDW contains high amounts of Ca and Fe, therefore reacts on hydration products mainly in C3S and C4AF improving the resistance of concrete against chloride ions.
![]()
Figure 1. Production process of Electrochemical Manganese Oxide (EMD) [19] [20] .
![]()
Table 1. Chemical analysis of steel reinforcement.
![]()
Table 2. Chemical composition of EMD and EMDW by-product.
1All metals are in the form of sulfate compounds e.g gypsum, Alunite, Jarosite; 2The percentage of 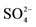 was not calculated from TOSOH HELLAS A.I.C industry.
was not calculated from TOSOH HELLAS A.I.C industry.
The specimens were prepared by mixing cement, calcite sand and water from supply network in ratio 1:3: 0.65. The Portland cement used were CEM I 42.5N and the chemical composition presents in the Table 3. The mortar specimens contained 5% w. w and 10% w. w by-product (EMDW) used as a replacement of cement. The mineral additive that was used consists mainly of alunite, yarosite, gypsum and sulphate salts of heavy metals.
The steel rebars were cleaned before installation into the mortars according to ISO/DIS 8407.3 [22] and weighted to a 0.1 mg accuracy. The specimens remained molded for 24 h and after removing the cast they were fully immersed into water for 7 days under laboratory conditions. A copper wire was enwrapped to each steel rebar to obtain electrochemical measurements. The dimensions and shape of the test specimens are shown in Figure 2. The corrosive environment and the conditions of exssperiments are given in Table 4.
The compressive strength of concrete was measured in cubic concrete specimens (15 × 15 × 15 cm3). The molds were filled with fresh concrete and vibrated for consolidation using a vibrating table with frequency 3000 Hz. For preparing of specimens were used calcite aggregates, Greek Portland cement CEM I 42.5 N and water of Attica network supply. The specimens after being demolded were placed in water in curing room (RH > 98%, T = 20˚C ± 1.5˚C) for 7 days. Three groups of samples were developed. The first group with aggregates and OPC (reference), the second group including 5% EMDW and the third group with 10% EMDW. The ratio of mixing materials of concrete is presented at the Table 5.
The specimens were constructed in the laboratory of reinforced concrete at TITAN cement plant. The mixture proportions and the characteristics of concrete are summarized in Table 6.
2.3. Methods
2.3.1. Electrochemical Measurements
• Linear Polarization Resistance (LPR) technique for measurement corrosion rate, corrosion current (i), and pola-
![]()
Figure 2 . Schematic representation of reinforced mortar specimen.
![]()
Table 3. Chemical analysis and mineralogy of Portland cement.
![]()
Table 4. Corrosive environment and conditions.
![]()
Table 5. Ratios of materials of concrete specimens for testing of compressive strength.
rization Resistance (RP) according with standard ASTM G59-97 [23] . The technique was developed based on Stern-Geary equation [24] . Tests were conducted using a Potentiostat/Galvanostat Model 263A from EG & G Princeton Applied Research and an associated software package to analyze the obtained data. The Steel rebars represented the working electrode, calomel was the reference electrode and a carbon bar was the counter electrode. The potential scan range was ±15 mV from OCP and the scan rate was 0.1 mV/s.
• Half-cell Potential (HCP) was measured for a period 24 months using calomel reference electrode (SCE) according with Standard ASTM C876-87 [25] . The measurements were initially recorded on an everyday basis for a period 10 days and then they were recorded on a weekly basis.
2.3.2. Carbonation Depth
The specimens were breaking and spraying the fresh cut sample with a pH indicator (1% w. w phenolphthalein
![]()
Table 6. Mixture proportions and concrete characteristics.
in ethanol) according with the method RILEM CPC-118 [26] . The specimens were exposed at the atmosphere conditions for 24 months and they were breaking every 6 months.
2.3.3. Gravimetric Mass Loss Measurements
To evaluate corrosion of steel reinforcement from chloride ions, mortar specimens were broken every 6 months. The steels were cleaned and de-rusted according to ISO/DIS 8407.3. The percentage of mass loss and Corrosion Rate (CR) were calculated from the following equations:
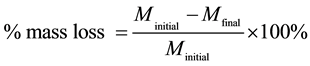 (1)
(1)
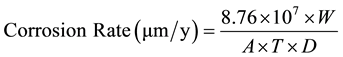 (2)
(2)
where Minitial is the mass of steel at the beginning, Mfinal is the mass after immersion in ΝaCl 3.5% w. w, W is the metal mass loss (g), A is the rebar’s surface (cm2), T is the time of exposure (h) and D is the density of steel (7.8 g/cm3).
2.3.4. Compressive Strength and Slump of Concrete
The slump of fresh concrete and compressive strength were measured according to ASTM C-143 [27] and BS EN 12390 - 3 [28] standards respectively. The measurements were performed at 7 and 28 days after curing in tanker with water. The loading rate of compression was 13.5kN/sec and the max load of machine was 3000 KN.
2.3.5. Total & Capillary Porosity of Concrete
• Total porosity of cement mortars. Porosity and pore size of mortar specimens were determined by Mercury intrusion Porosimetry (MIP). MIP method is based on the Washburn [29] equation:
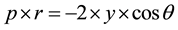
where r is the radius of the pore where mercury intrudes, γ surface tension of mercury and θ the contact angle of the mercury on the surface of a solid sample. The total porosity was measured using Carlo Erba 2000 Mercury porosimeter. The mortars exposed to the atmosphere for 18 months and they were broken at 6 and 18 months.
• Capillary porosity of concrete. Determination of open porosity described by many authors [30] [31] as follows: Initially, the samples are dried in an oven (at 105˚C) to remove the majority of physically-bound water. They were then placed in an airtight container and the pressure was lowered gradually to 2000 Pa using a vacuum pump for 3 h. Once the samples were immersed, the liquid supply was stopped and the specimens were kept immersed for the next 24 h. During this time, the absolute air pressure in the container did not exceed 2000 Pa. The vessel was then returned to atmospheric pressure and the specimens were left immersed for another 24 h.
From the mass of the liquid-saturated sample Mw and the mass of the immersed liquid-saturated sample Ma, the volume V of the sample was determined:
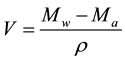
where ρ is the density of water. The open porosity was calculated by:
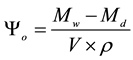
where Μd is the mass of the dry sample.
3. Results and Discussion
The Half-cell potential (Ecorr) shows, the tendency of steel for corrosion and does not reflected the corrosion rate of reinforcement which is affected by number of factors such as the diffusion of oxygen, concrete’s porosity and the presence of highly resistive layer [32] . The Table 7 appears the corrosion condition of steel relative to corrosion potential (Ecorr).
In Figure 3, are presented the corrosion potentials versus exposure time for cement mortars specimens with
5% w. w and 10% w. w replacement of cement with EMDW and immersion in 3.5% w. w NaCl solution for a period of 24 months. The reference specimens were prepared for comparison reasons and the ratio of cement:sand:water was 1:3:0.65. A saturated Calomel electrode (SCE) was used as reference electrode.
At the beginning of immersion, reference specimens have higher values of corrosion potential than other two categories. After two months of exposure reference have almost the same Ecorr with 5% EMDW but the mortars with 10% addition have the most electronegative values due to high porosity. The values of corrosion potentials all of categories of specimens after 24 months partial immersion in ΝaCl solution are between −600 mV to −400 mV and according to Broomfield the corrosion of steel is severe. The presence of chloride ions and the high relative humidity, corrode the steel reinforcement and decrease the values of Ecorr.
Figure 4 presents the corrosion potentials in fuction of time, for exposed specimens in atmosphere for 24 months. In contrast the previous chart, the corrosion potentials values are presented less electronegatives. From the graph seems that the corrosion potentials ranging between −300 mV and +150 mV and therefore the steel reinforcements are in passive condition and trend does not corrode according to ASTM C876-87.
![]()
Figure 3. Corrosion potential for reinforced mortars immersed in 3.5% w. w NaCl solution.
![]()
Figure 4. Corrosion potential (mV) for reinforced mortars exposed in atmosphere.
![]()
Table 7. Corrosion condition according (Broomfield, 2003).
The corrosion current (icorr) was measured every two months with LPR method and the theoritical mass loss is calculated by Faraday’s law. From Farady’s law:
![]()
where, m is the theoretical mass loss (g), t is the time (s), i is the current (A), M is the atomic weight of iron (55.847 g/mol), z is the ion chance (for Fe is 2), F is the Faraday’s constant (96.487 A×s).
Figure 5 is presented the results of theoritical mass loss measurements. After 7 months of partial immersion in NaCl 3.5% w. w, the embedded steels of mortars with EMDW appear lower values of mass loss compared with reference. At the end of measurements, the reference specimens have 49% higher value from 5% w. w EMDW and 32% from 10% w. w EMDW. High concentrations of calcium and iron of EMDW contributes to the hydration of cement reducing the permeability and porosity of concrete (Figure 10). Based on other studies [33] LPR and HCP are the most suitable methods of detection of steel depassivation and the most influence factors of chloride threshold values is the steel-concrete interface and the steel potential. Analysis of theoritical mass loss by LPR method and gravimetric mass loss show that the replacement of cement up to 10% decreased the corrosion of steel embedded in concrete.
Figure 6 is presented the carbonation depth (d) versus time of cement mortars exposed in atmosphere. From this graph seems that the addition of EMDW decrease the carbonation depth in comparison with reference after 24 months exposure to the atmosphere. The carbonation depth was calculated according the function [34] :
![]()
where, d is the carbonation depth in mm, t is the exposure time in yrs., Κ is the diffusion coefficient of CO2 in mm/yr1/2.
The mass loss of steel embedded in cement mortars is shown at the Figure 7. The specimens with 10% EMDW have the lowest values of mass loss compared to the other two categories of specimens. The low values of mass loss due to the decrease of the porosity of specimens with the additive (Figure 10).
The Corrosion Rate (CR) of steels, calculated according with the function (2). The results of measurements are shown to the Figure 8. Corrosion rate increases to all categories of specimens for a period of 24 months. The replacement of cement by 5% and 10% EMDW decrease the corrosion rate against chloride ions after 24 months partial immersion in 3.5% w. w NaCl solution compared with reference.
The slump and the density of concrete are given in Table 8. The replacement of cement by EMDW was 5% and 10% and the ratio W/C = 0.57. Figure 9 illustrates the compressive strength of concrete at 7 and 28 days after curing in water for 7 days. Τhe specimens with 10% additive have slightly increased compressive strength while the other two categories almost similar. The reduction of total porosity (Figure 10) leads to increased compressive strength.
The porosity was measured by mercury intrusion Porosimetry technique and illustrates in Figure 10. After 18 months of exposure to the atmosphere C the specimens have the same percentage of porosity. The porosity of mortars with 10% EMDW was reduced 53, 5% and reference was reduced 35%.
The results of open porosity measurements of concrete after 130 days exposure to chloride solution is given in Figure 11. Three groups of specimens were made. Two groups with replacement of cement by 5% and 10% EMDW and one group without additive (reference) for comparing reasons. The specimens were cubic (100 × 100 × 100 mm3), and they were prepared by calcite aggregates, CEM I 42.5N, water from Nicosia supply network and ratio W/C = 0.65.
![]()
Figure 5. Theoretical mass loss (g) calculated by Faraday’s law versus time.
![]()
Figure 6. Carbonation depth (mm) of specimens exposed in atmosphere.
![]()
Figure 7. Mass loss (%) of specimens exposed in atmosphere.
![]()
Figure 8. Corrosion rate (μm/y) of steel in NaCl 3.5% w.w solution.
![]()
Figure 9. Compressive strength (fc) of concrete (MPa).
![]()
Figure 10. Total porosity (%) of cement mortars exposed in atmosphere versus time by Mercury Intrusion Porosimetry method.
![]()
Figure 11. Open porosity (%) of concrete specimens in NaCl 3.5% w. w solution by vacuum saturation method.
![]()
Table 8. Density measurements (kg/cm3) and slump (mm).
In all cases the porosity decreased after 28 days of immersion in sodium Chloride solution. The additive containes Alunite and Jarosite and according to Katsioti et al. [35] the addition of jarosite-alunite up to 1% has no influence on the formation of the mineralogical phases but high content resulted in a higher ferrite proportion. Also, Chloride ions react with hydration products and generating Friedel’s salt [36] . Based on the above, the high contents of calcite and iron of additive causes the reduction of porosity.
4. Conclusions
The following conclusions can be deduced from the present study:
• The replacement of cement by EMDW appears to significantly improve the durability of concrete against chloride ions;
• The addition of EMDW decreases the carbonation depth after 24 months of exposure in atmospheric conditions. High levels of Calcium and Iron increase the creation of C3S and C4AF which reduce the porosity and the diffusion of CO2;
• The compressive strength of specimens with 10% additive appears better than reference at early and late ages due to reduce permeability of concrete;
• The specimens with addition EMDW have lower values of gravimetric and theoretical mass loss than reference after partial immersion in 3.5% w. w NaCl solution;
• The total porosity of composite mortars exposed in atmosphere decreased more than specimens without EMDW addition;
• The use of additive reduced the open porosity more than reference from 70 days and then;
• The addition of EMDW decreased the drying shrinkage. Between 7 and 28 days, the density of reference was reduced by 11 kg/m3 and the specimens with 5% and 10% EMDW, 2 kg/m3 and 5 kg/m3 respectively.