Fermentative Production of Mycelial Chitosan from Zygomycetes: Media Optimization and Physico-Chemical Characterization ()
1. Introduction
Chitosan is a natural amino polysaccharide comprising copolymers of D-glucosamine (GlcN) and N-acetyl- D-glucosamine (GlcNAc), and is a deacetylated derivative of chitin—the second most abundant natural polymer after cellulose [1] . It is polycationic, nontoxic, biodegradable as well as antimicrobial and has been reported to have numerous applications especially in food, pharmaceutics and cosmetics [2] - [4] .
Commercially available chitosan produced by chemical deacetylation of chitin has several disadvantages as isolation of chitin from crustacean shells and its conversion to chitosan requires strong alkali treatment, high temperature, and a long processing time, which make this process costly [5] . Further, multiple chemical treatments cause an increase in the level of environmental pollution [6] .
Recent advances in fermentation technology addressed that many of these problems can be overcome by culturing chitosan-producing fungi, particularly Zygomycetes species, which are known to contain chitosan as natural cell wall component [7] - [10] .
Chitosan in cell walls produced through enzymatic deacetylation of chitin chain through the action of N-dea- cetylation is a common step in the modification of sugar chains [11] . Mycelial chitosan offers significant advantages; the most important is like where crustacean waste supplies are limited by seasons and sites of fishing industry; fungal mycelium can be obtained by convenient fermentation process with organisms that can be readily cultured on cheap nutrients and the cell wall material can be recovered by simple chemical procedures. Fungal mycelia have lower level of inorganic materials compared to crustacean wastes, and thus no demineralization treatment is required during the processing. Furthermore, the mycelial chitosan is free of heavy metal contents such as nickel and copper [12] [13] . Crustacean chitosan may vary in the physico-chemical properties, while mycelial chitosan has relatively consistent properties [12] - [16] .
Insight of the above advantages, the objective of present work is mainly focused to screen different fungal strains and to optimize culture conditions using one-factor-at-a-time and statistical analysis by response surface methodology (RSM) based on the Box-Behnken design for the highest production of mycelial chitosan by submerged fermentation. RSM was employed to build models, to evaluate the effective factors and their interaction and to select optimum conditions, with a minimum number of experiments. Furthermore, characterization of physical properties of produced mycelial chitosan was investigated.
2. Materials and Method
2.1. Micro-Organism and Its Maintenance
Five different strains of Zygomycetes, namely, Absidia blakesleeana NCIM 889, Absidia butleri NCIM 977, Cunninghamella blakesleeana NCIM 687, Cunninghamella echinulata NCIM 691 and Rhizopus oryaze NCIM 1009 were purchased from National Collection of Industrial Microorganism (NCIM) Pune, India. For the screening, all the five strains were grown and maintained on potato dextrose agar (PDA) slants, grown at 30˚C for 10 days and stored at 4˚C and subcultured on a monthly basis. The chemicals and media components utilized in the present study were procured from Hi-Media (Mumbai, India). Concentrated NaOH solution was commercial grade and all chemicals were of analytical grade. Standards of chitosan were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
2.2. Medium and Culture Conditions
The inoculum was prepared in Erlenmeyer flasks (250 ml) containing 100 ml of potato dextrose broth, inoculated with spore suspension (1.8 × 108 spores/ml) and incubated at 30˚C for 16 h on a rotatory shaker (180 rpm). A 7.5% (v/v) of sixteen hr old inoculums was added aseptically to the 500 ml Erlenmeyer flasks, each containing 200 ml of the sterile production media of glucose 2%, peptone 1%, yeast extract 0.1%, (NH4)2SO4 0.5%, K2HPO4 0.1%, NaCl 0.1%, CaCl2 H2O 0.01%, MgSO4·7H2O 0.05% [12] . The pH of production media was adjusted to 4.5 using hydrochloric acid. All inoculated flasks were kept on a rotary shaker at 30˚C ± 2˚C and 180 rpm. Various strains belonging to Zygomycetes species were screened for production of mycelial chitosan. The concentration of various carbon and nitrogen sources and culture conditions varied according to the experimental design described below. Unless otherwise stated, the cultivation was carried out using 500 ml Erlenmeyer flasks on a 180 rpm shaker at 30˚C ± 2˚C. All procedures were performed in triplicate.
2.3. Optimization of Physical Parameters Using One-Factor-at-a-Time Approach
To determine the optimum batch time for mycelial chitosan production, its production was carried out under the defined conditions, for different time periods ranging from 12 to 96 h. In order to investigate effect of initial pH of production media, fermentation runs were carried at initial pH varying from 3 to 6. The pH was adjusted using 0.1 N hydrochloric acid or 0.1 N sodium hydroxide. The effect of temperature on biomass and yield of mycelial chitosan was studied by incubating the production medium at varying temperature from 25˚C to 30˚C. In all three parameters after incubation, biomass was harvested, filtered through Whatman filter paper no. 1, washed with distilled water and dried them at 60˚C to a constant weight to determine the cell dry weight.
2.4. Effect of Different Carbon Sources on Mycelial Chitosan Yield
Several carbon sources were used to study the effect on mycelial chitosan production. To study the effect of various carbon sources on chitosan production, in the production medium, D (+) glucose was substituted with eight different carbon sources. All the carbon sources were used at 2% (w/v) concentration. Rests of the physicochemical parameters were constant.
2.5. Effect of Different Nitrogen Sources on Mycelial Chitosan Yield
To evaluate the effect of different nitrogen sources on mycelial chitosan production, combination of peptone (1% w/v), yeast extract (0.1% w/v) and ammonium sulphate (0.5% w/v) was replaced with different nitrogenous sources (both organic and inorganic) with concentration of 1.6% w/v. Mycelial chitosan yield and dry cell weight (DCW) were determined and rest of the physicochemical parameters were constant.
2.6. Experimental Design and Data Analysis
The most influential factors for mycelial chitosan production found by “one-factor-at-a-time” approach were further optimized with statistical approach. To describe the level of the significant parameters, interaction between variables, which influence the yield of mycelial chitosan and, the nature of the response surface in the optimum region, a Box-Behnken design [17] , and the response surface methodology was performed.
2.7. Box-Behnken Design
In this study, the experimental plan consisted of 17 trials and the independent variables are studied at three different levels, low (−1), medium (0) and high (+1). The variables and their coded levels used for the study are shown in Table 1. All the experiments were done in triplicate and the average of mycelial chitosan yield obtained was taken as the dependent variable or response (YCY). The second-order polynomial coefficients were calculated and analyzed using the Design Expert version 7.0.0 (STAT-EASE Inc., Minneapolis, MN, USA), statistical package.
The general form of the second degree polynomial Equation (1) is
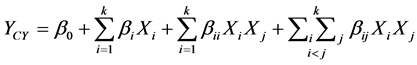 (1)
(1)
where, YCY is the predicted response; β0 a constant; βi the linear coefficient; βii the squared coefficient; and βij the cross-product coefficient, k is number of factors, xi and xj the level of the independent variables, subscripts i and j takes values from 1 to the number of variables.
In the present study, three variables are involved and hence n takes the value 3. Thus, by substituting the value 3 for n, and the coded variables for natural ones, Equation (1) becomes the following form: The yield of mycelial chitosan was taken as dependent variable or response YCY, and a multiple regression analysis of the data was carried out for obtaining an empirical model that relates the response measured to the independent variables.
![]()
Table 1. Experimental range, level and code of independent variables.
2.8. Statistical Analysis of Data
The statistical and regression analyses of experimental data obtained from Box-Behnken design was done by using software Design Expert version 7.0.0 (STAT-EASE Inc., Minneapolis, MN, USA) to determine the significant differences (p ≤ 0.05) in response under different conditions. Three-dimensional surface plots were constructed for visualization of interaction between significant variables and their optimal values. The goodness of fit of the model was evaluated by the coefficient of determination (R2) and the analysis of variance (ANOVA) and its statistical significance was checked by an F test quadratic polynomial equations were attained by holding one of the independent variances at a constant value and changing the level of the other variables.
2.9. Extraction of Mycelial Chitosan
The dried fungal cell mass was finely homogenized and subjected to alkali treatments to extract alkali soluble material like glucan and protein, present in fungal biomass. Dried mycelia homogenized dry cell mass were treated with 1 N sodium hydroxide solution (1:40, w/v) carried out by autoclaving biomass at 121˚C for 20 min. The alkali insoluble materials (AIM) were centrifuged (10,000 rpm, 15 min), washed with distilled water, until it completely gets neutralized and further treated with 2% acetic acid (1:40, w/v) at 95˚C for 6 h, closely following the method of [18] . Through centrifugation, the supernatant, containing the chitosan, was isolated from the acid insoluble fraction. The mycelial chitosan was precipitated from supernatant by adjusting pH 10.0 with 2 M sodium hydroxide. The precipitate was centrifuged (10,000 rpm, 15 min) and washed with distilled water to pH 7, followed by 95% (v/v) ethanol (1:20, w/v) and acetone (1:20, w/v), and dried at 60˚C for 24 h and, weighed to calculate a mycelial chitosan yield. According to McGahren et al. [19] the alkali-insoluble precipitate obtained after suitable alkali and acid extraction contained virtually pure mycelial chitosan.
2.10. Characterization of Mycelial Chitosan
Isolated mycelial chitosan obtained by submerged fermentation by evaluating some of the physical properties including glucosamine content, Fourier-transform infrared (FTIR) spectroscopy, degree of deacetylation (DD), viscosity and molecular weight (MW).
2.10.1. Determination of Glucosamine Content
Determination of glucosamine content of mycelial chitosan was based on colorimetric measurement. Chitosan samples after extraction were hydrolyzed with 2 M hydrochloric acid at 110˚C for 2 h and the liberated D-glucosamine was deaminated with nitrous acid to yield 2,5-anhydromannose which react with 3-methyl-2- benzothiazolinone hydrazone hydrochloride (MBTH) to produce intense blue-colored complex measured at 650 nm [20] .
2.10.2. Infra Red Spectroscopy
The structure of extracted mycelial chitosan was confirmed by infra red spectroscopy using KBr pellet method in FTIR (Perkin Elmer Model 1600 Series, MA, USA). In FTIR spectra were recorded in the middle infrared (4000 cm−1 to 400 cm−1) with a resolution of 4 cm−1 in the absorbance mode for 16 scans at room temperature. The mycelial chitosan samples were prepared by grinding the dry mycelial chitosan powder with powdered KBr, in the ratio of 1:5 (sample:KBr) and then compressed to form KBr pellet and subjected to FTIR analysis [21] .
2.10.3. Degree of Deacetylation
The degree of deacetylation (DD) was determined using the concept of baseline method [22] . The IR spectrum recording procedure is same as described above. According to the IR spectrum, the DD was calculated by measuring the absorbance ratio of A1655 and A3450 [23] . The amide I band at 1655 cm−1 and the hydroxyl group absorption band at 3450 cm−1 were used as internal reference. An equation proposed by Baxter et al. for determination of degree of deacetylation is as follow:
 (2)
(2)
2.10.4. Determination of Viscosity and Molecular Weight
Viscosity of mycelial chitosan was determined with an Ubbelohde-type capillary viscometer [24] . Chitosan solution (1% v/v) was prepared in 2% (v/v) acetic acid, stirred for 4 h and filtered to remove insoluble materials. After that, the solution was allowed to stand to remove air bubbles. Measurements were made in triplicate on each sample at 25˚C. Values were reported in dL/g. The running times of the solution and solvent were recorded as seconds (sec) and used to calculate intrinsic viscosity [ηin]. The reduced viscosity (ηred), which is specific viscosity (ηsp)/concentration (C), as tabulated, (ηred = ηsp/C) where (ηsp = ηrelative − 1), and
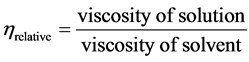 (3)
(3)
Relative viscosity (ηrel) = specific viscosity (ηsp) = ηrel − 1
Reduced viscosity (ηred) vs. concentration of chitosan solution (g/dl, %) were plotted on a graph. The intrinsic viscosity ([ηin], dl/g) was obtained by extrapolating reduced viscosity vs. concentration data to zero concentration.
Using this value of the intrinsic viscosity ([ηin], dl/g) average molecular weight of mycelial chitosan (Mw) was determined. The viscosity-average molecular weight of mycelial chitosan solutions were calculated using the Mark Houwink equation which provides the relationship between intrinsic viscosity and molecular weight [25] .
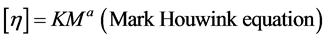 (4)
(4)
where, “K” and “a” are constants for given solute-solvent system and temperature.
3. Results and Discussions
A process of screening is necessary to determine the most influential factors affecting the cultivation process. In order to obtain the suitability of fermentation conditions the effect of various strains, incubation period, pH, temperature and various carbon and nitrogen sources on biomass and mycelial chitosan yield, was investigated.
3.1. Screening of Different Fungal Strains
Screening of fungal strains for mycelial chitosan producers are presented in Figure 1. Maintaining the fermentation parameters same, DCW of the strains ranges from 3.6 g/L (R. oryzae NCIM 1009) to 6.8 gm/L (A. butleri NCIM 977). In our screening among five fungal strains, we found that A. butleri NCIM 977 gave maximum production of mycelial chitosan (401 mg/L) followed by C. echinulata NCIM 691 (372 mg/L) and R. oryaze NCIM 1009 (274 mg/L). According to Bartnicki-Garcia [7] Zygomycetes species, contains chitosan as natural components of their cell wall. Nwe et al. [11] suggested that chitosan biosynthesis starts with production of chitin with the conversion of glucose into N-acetylglucosamine-1-phosphate, which reacts with UTP to form UDP- N-acetylglucosamine in a reaction catalyzed by glucosamine-6-phosphate synthase. Subsequently, the enzyme chitin synthase transfer the sugar dimer of N-acetylglucosamine moiety into the growing chitin polymer chain. Furthermore in Zygomycetes, deacetylation of the growing chitin chain occurs through the action of the enzyme chitin deacetylase which results in formation of mycelial chitosan. From the present experiment, it was found that A. butleri NCIM 977 was the most suitable fungal strain since it produced highest mycelial chitosan and therefore used for further optimization study.
3.2. Screening of Physical Parameters and Nutrients by One-Factor-at-a-Time Method
In order to determine the optimum batch time for mycelial chitosan production, fermentation process was carried out in 200 ml of sterile production media at 30˚C ± 2˚C on a rotary shaker at 160 rpm for different time level ranging from 12 h to 96 h. It was observed that 72 h time period resulted into maximum mycelial chitosan production of 585.33 mg/L with DCW 7.6 g/L (Figure 2). Further increase in fermentation time observed low productivity and biomass concentration which may be due to physiological changes in the fungal cell wall [19] . Rane and Hoover [26] reported that decrease in mycelial chitosan yield is due to its degradation by chitosanase activity. Chitosan is produced in the fungal cell wall by deacetylating its precursor, nascent chitin [12] . During the exponential phase, the ratio of free chitosan molecules is relatively high, due to the active growth. Once the
![]()
Figure 1. Screening of fungal strains for mycelial chitosan production by A. butleri NCIM 977.
![]()
Figure 2. Effect of fermentation batch time on mycelial chitosan production by A. butleri NCIM 977.
culture enters the stationary growth phase, more of the chitosan is anchored to the cell wall of the Zygomycetes by binding to chitin and other polysaccharides and extraction becomes more difficult [7] . Therefore, although the fungal biomass was increased during the stationary growth phase, less chitosan is obtained while maximum mycelial chitosan yields at the late exponential phase [12] .
3.3. Effect of Different Initial pH Values on Production of Mycelial Chitosan Yield
The pH of the medium always influences the physiology of a microorganism by affecting nutrient desirability, enzyme activity, oxidative-reductive reactions and most importantly cell membrane morphology. Among the various initial pH range studied (3 - 6), an initial pH 5.5 supported the maximum production of mycelial chitosan (669.16 mg/L) as well as biomass (Figure 3). The results were in agreement with the studies reported by Synowiecki and Ali-Khateeb [18] for Mucor rouxii while Yokoi et al. [27] for Gongronella species and Muzzarelli et al. [28] for Absidia coerulea for mycelail chitosan production. This may be due to the fact that the pH ranging from 4.5 to 5.5, favors the production of enzyme chitin deacetylase, which convert chitin to chitosan in fungal cell wall [13] .
3.4. Effect of Different Effect of Temperature on Production of Mycelial Chitosan Yield
Temperature influences the metabolic activities and microbial growth which affects the production. In order to find out the effect of temperature on mycelial chitosan production, fermentation was carried out at different temperatures ranging from 20˚C to 35˚C. The yield increases from 20˚C till 30˚C and further decreased at 35˚C (Figure 4). A maximum mycelial chitosan production of 667.16 mg/L with highest biomass production (8.4 g/L) was observed at 30˚C.
3.5. Effect of Carbon Sources on Production of Mycelial Chitosan Yield
During microbial fermentations, the carbon source not only acts as a major constituent for building of cellular material, but also as an important energy source. All carbon sources with 2% (w/v) concentration showed different effects on mycelial chitosan production (Figure 5). The fungal strain A. butleri NCIM 977 grown on the medium supplemented with fructose, sucrose, galactose resulted in increase biomass production however glucose as a sole carbon source supported the highest biomass as well as mycelial chitosan production as compared to other carbon sources. Among all carbon sources glucose employed showed maximum mycelial chitosan production (683.16 mg/L) with DCW of 8.3 g/L, followed by galactose (383 mg/L), fructose (343.33 mg/L), and sucrose (291.5 mg/L). It may be due to the fact that glucose can be easily assimilated in the metabolic pathway for biosynthesis.
![]()
Figure 3. Effect of initial pH on mycelial chitosan production by A. butleri NCIM 977.
![]()
Figure 4. Effect of temperature on mycelial chitosan production by A. butleri NCIM 977.
![]()
Figure 5. Effect of carbon sources on mycelial chitosan production by A. butleri NCIM 977.
3.6. Effect of Nitrogen on Production of Mycelial Chitosan Yield
The nitrogen source is a critical factor which needs to be optimized for mycelial chitosan production. Chitosan is a nitrogen containing biopolymer, which is deacetylated form chitin. Fungi require an inorganic or organic nitrogen source as nutrient to synthesize the chitin and chitosan for their cell wall. Hence the nitrogen source is one of the important factors for the production of chitosan by fungi [29] . Among the organic and inorganic nitrogen sources, organic nitrogen source exhibited a prominent effect on mycelial chitosan production (Figure 6 and Figure 7). Among the organic nitrogen sources studied soyabean meal produced the highest DCW of 11.13 g/L followed by beef extract (7.44 g/L) and yeast extract (7.06 g/L). However, tryptone supported the maximum production of mycelial chitosan (570.33 mg/L) with DCW of 6.78 g/L. In the absence of nitrogen source, chitosan yield was found to be drastically decreased, hence it can be concluded that that nitrogen source is significant parameter in the fermentative production of mycelial chitosan.
![]()
Figure 6. Effect of organic nitrogen sources on mycelial chitosan production by A. butleri NCIM 977.
![]()
Figure 7. Effect of inorganic nitrogen sources on mycelial chitosan production by A. butleri NCIM 977.
3.7. Optimization of Culture Conditions by Box-Behnken Design
In this study, according to Box-Behnken design, all 17 designed experiments were conducted with different combinations of three independent parameters (glucose, tryptone and yeast extract) at three different levels, low (−1), medium (0) and, high (+1) to study the combined effects of these factors towards yield of mycelial chitosan. The variables and their coded levels used for the study are shown in Table 1. Box-Behnken design matrix of independent variables in coded units along with experimental and predicted values for mycelial chitosan production is shown in Table 2. The results were analyzed by multiple regression analysis. Maximum yield of mycelial chitosan (1 g per litre of fermentation media) was recorded under the experimental conditions having media
![]()
Table 2. Box-Behnken design matrix with experimental and predicted values of chitosan production by A. butleri NCIM 977.
aValues are mean ± SD of three determinations.
concentrations of glucose 1.5% (w/v), tryptone 1.6% (w/v), and yeast extract 1.1% (w/v). Consequently, the following second order polynomial equation for mycelial chitosan production was regressed and expressed in term of coded factors. It represents yield of mycelial chitosan (YCY) as a function of concentration of glucose (A), tryptone (B) and yeast extract (C).
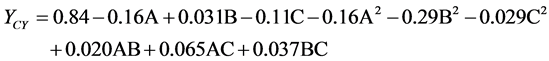 (5)
(5)
In order to determine whether or not the quadratic model is significant, it is necessary to conduct analysis of variance (ANOVA). ANOVA for response surface quadratic model is presented in Table 3. The p-values were used as a tool to check the significance of each coefficient, which, in turn are necessary to understand the pattern of the mutual interactions between the test variables. The lower p-values are the more significant and effective in process parameters [30] . Here, the p-value of the model was smaller than 0.0001, which indicated that the model was suitable for use in this experiment. The lack of fit test, measures the failure of the model to represent experimental data in the experimental domain at point which are not included in regression analysis [31] . The p-value of “lack of fit” was 0.6031 (p > 0.01), indicated that “lack of fit” was insignificant relative to the pure error and the model equation was adequate to predict yield of mycelial chitosan under any sets of combination of variables. The regression coefficients and the corresponding p-values were presented in Table 4. From the p-values of each model term, it could be concluded that two linear coefficients (A, C), two quadratic coefficients (A2, B2) and one cross-product coefficients (AC) were significant. It implies that three parameters are very critical for the production of mycelial chitosan and there was strong interaction between concentrations of glucose and yeast extract. The statistical significance of the polynomial equation was carried by F-test. The model F-
![]()
Table 3. Analysis of variance (ANOVA) for the fitted quadratic polynomial model of mycelail chitosan productiona from the results of Box-Behnken experimental design.
aDegree of freedom; *Significant; **Not significant.
![]()
Table 4. Regression coefficients and their significance of the quadratic model of mycelial chitosan production by A. butleri NCIM 977.
A—Glucose; B—Tryptone; C—Yeast extract; aDegree of freedom; *Significant; **Not significant.
value of 64.20 (p < 0.0001) implies the model is significant and is calculated as ratio of mean square regression and mean square residual. The goodness of fit of the model was checked by coefficient of determination (R2) and adjusted coefficient of determination (adjusted R2). The R2 value varies from 0 to 1.0 and close to 1.0 implies better accuracy of the model. But in certain cases higher R2 value may be resulted in presence of large number of insignificant variables in the model and thereby predicts poor response. So the term adjusted R2 was introduced which corrects R2 value accordingly to the sample size and number of terms in model. Ideally adjusted R2 should be close to R2 value. Larger difference between R2 and adjusted R2 gives warning that model content too many insignificant terms [32] . The high R2 value (0.9880) indicates, good correlation between experimental and predicted values and 98.80% of the variability in the response could be explained by the model. The R2 of 0.9880 is in reasonable agreement with the adjusted R2 of 0.9726 confirming the validity of the model. “Adequate Precision” measures the signal to noise ratio and its value can be predicted by statistical analysis. A ratio greater than 4 is desirable [32] . In the present study, a ratio of 27.907 indicates an adequate signal. This explains that the model can be used to navigate the design space. The coefficient of variation (CV) is the ratio of the standard error of estimate to the mean value of the observed response, expressed as a percentage. Usually, the higher the value of CV, the lower is the reliability of experiment while low CV value predicts accuracy and reliability of the experiments conducted [33] . Here CV value was found to be 6.13 indicates a greater degree of precision. This is also evident from the parity plot (Figure 8), obtained by plotting observed and predicted yield of mycelial chitosan and it suggests satisfactory correlation between experimental and predicted value, as the data points were confined close to the digonal line, showing that the prediction of experimental data is quite satisfactory.
Perturbation graph shows the effect of each of the independent variables on response while keeping other variables at their respective zero level. From Figure 9 it is revealed that tryptone concentration (B) was most critical factor followed by glucose concentration (A) whereas yeast concentration (C) is least influential factor for the production of mycelial chitosan.
Accordingly, 3-D response surface plot were generated for the pairwise combination of two independent variables where remaining variables are fixed at their respective zero level and described by the regression model which illustrate the interactive effect of this independent variables on the response variable and also determine optimum level of each variable [34] .
Figure 10(a) represents the effects of glucose concentration, tryptone concentration and their reciprocal interactions on yield of mycelial chitosan at constant yeast extract concentration of 1.6% (w/v). At the designed
![]()
Figure 8. Parity plot for actual and model predicted values for mycelial chitosan production.
![]()
Figure 9. Perturbation graph showing the effect of each in dependent variable on mycelial chitosan production while keeping other variables at their respective zero level.
![]() (a)
(a)![]() (b)
(b)![]() (c)
(c)
Figure 10. (a) Response surface plots showing relative effect of two variables glucose and tryptone; (b) Response surface plots showing relative effect of two variables glucose and yeast extract; (c) Response surface plots showing relative effect of two variables tryptone and yeast extract.
range of glucose concentration from 1.5% (w/v) to 2.5% (w/v), the amplification of mycelial chitosan yield resulted in a linear increase in tryptone concentration, and then reduced. It can be inferred that yield of mycelial chitosan was markedly affected by the combination of glucose concentration and tryptone concentration. An increase of tryptone concentration increased the mycelial chitosan yield at a constant glucose concentration within a tryptone concentration of 1.6% (w/v). The appropriate maximal mycelial chitosan yield was determined at a glucose concentration of 1.5% (w/v). Figure 10(b) shows the effects of glucose concentration, yeast extract concentration and their reciprocal interactions on mycelial chitosan yield at a tryptone concentration of 1.6% (w/v). At the designed range of glucose concentration from 1.5% (w/v) to 2.5% (w/v), the yield of mycelial chitosan increased with decreasing concentration of yeast extract. At low concentration of yeast extract, the yield of mycelial chitosan was found to be maximum. It could be seen from Figure 10(b) that at high concentrations of both glucose and yeast extract the yield of mycelial chitosan was minimum while the optimum concentrations of glucose and yeast extract for maximum mycelial chitosan production was around 1.5% (w/v) and 1.1% (w/v) respectively. The effect of concentrations of tryptone and yeast extract on the yield of mycelial chitosan at a glucose concentration of 2% (w/v) is provided in Figure 10(c). At first, an increase in mycelial chitosan yield was observed with the increasing of yeast extract and tryptone concentration. But the trend was reversed when the concentrations of glucose and tryptone reached a certain value. It could be seen from Figure 10(c) that yield of mycelial chitosan was affected significantly by tryptone concentration.
In order to verify the predicted results, an experiment was performed using the optimized nutrient levels. The optimum conditions for these selected parameters were predicted using desirability function criteria available in design expert software. The maximum predicted mycelial chitosan production could be achieved with glucose concentration of 1.58% w/v (A), Tryptone concentration of 1.61% w/v (B), and yeast concentration of 1.11% w/v (C). Under these conditions experiments were carried out in triplicate. The mean value of the yield of mycelial chitosan was 1.002 mg/L, which agreed with the predicted value (1.0012 mg/L) well. As a result, the models developed were considered to be accurate and reliable for predicting the production of mycelial chitosan from A. butleri NCIM 977.
3.8. Characterizations
3.8.1. Determination of Glucosamine Content
Chitosan are linear polysaccharides consisting of N-acetyl-D-glucosamine and D-glucosamine units present in different ratios in the polymers. The glucosamine content in chitosan isolated from fungal source A. butleri NCIM 977 was determined to be 80.68%, a little more than the report of Synowieki and Al-khateeb [18] . The relatively high content of glucosamine showed that the product contained mainly aminosugars and its purity was up to 80.68%.
3.8.2. Infra Red Spectroscopy
IR spectroscopy has been reported as a relatively quick, simple technique and commonly used for qualitative and quantitative evaluations of chitin and chitosan characteristics, mainly their functional groups, the degree of acetylation/deacetylation and impurities [23] . The FTIR spectrum of mycelial chitosan from A. butleri NCIM 977 obtained from submerged fermentation was compared with standard chitosan are shown in Figure 11. Several bands have been proposed as internal reference bands of chitosan: the OH stretching band at 3450 cm−1; the C-H stretching bands within 2870 - 2880 cm−1; the skeletal vibrations involving the C-O-C stretching band at 1030 - 1070 cm−1; the -CH2 bending centered at 1420 cm−1; the anti-symmetric stretching of the C-O-C bridge around 1160 cm−1; 1315 - 1320 cm−1 (amide III band); 1620 - 1630 cm−1 (-NH bending of NH2); and 890 - 900 cm−1 (C-O-C bridge as well as glucosidic linkage) [25] . In present study, our results were also similar to those reported by Kasaai [25] and Khan [22] and similar to that of mycelial chitosan obtained from M. rouxii [18] . The most significant parts of these spectra are those showing the amide bonds at approximately 1655 and 1313 cm−1. Other functional group include hydroxyl stretching band at 3427 cm−1, amide I band at 1655, primary amine band at 1638 - 1561 cm−1 amide II band at 1561 cm−1 and amide III band at 1320 cm−1. The band at 897 cm−1 was referenced as glycosidic linkage of β-anomer. These absorption bands preliminary ascertained the product to be chitosan.
3.8.3. Degree of Deacetylation
IR spectroscopic method was commonly used for determination of DD value of mycelial chitosan. It was initially
![]()
Figure 11. FTIR spectra of (a) standard chitosan and (b) mycelial chitosan obtained from A. butleri NCIM 977cultivated on optimized formula.
proposed by Moore and Roberts. It has a number of advantages like relatively fast method and does not require dissolution of the chitosan sample in an aqueous solvent. IR spectroscopy is primarily a solid-state method utilizing the concept of baseline for DD calculation. The baseline proposed by Baxter et al. [23] was modified from the method reported by Domszy and Roberts [22] . In the present study, the DD value of mycelial chitosan obtained from submerged fermentation of A. butleri NCIM 977 was determined to be 79.89%. In present study DD value of mycelial chitosan was found to be slightly lower than the reported values of DD of chitosan from other fungi [13] [14] [35] .
3.8.4. Determination of Viscosity and Molecular Weight
The viscosity of mycelial chitosan obtained from the submerged fermentation of A. butleri NCIM 977 was 73.22 dl/g. The molecular weight of mycelial chitosan was depends and vary upon substrate, medium supplementation and fermentation mode. The viscosity average molecular weight of mycelial chitosan was calculated from Mark Houwink equation. In present study the molecular weight (MW) of mycelial chitosan was found to be 8.07 × 104 Da and was lower compared to commercial chitosan. Previous research has reported the MW to be between 3 × 104 Da - 1.4 × 105 Da [13] [36] . As compared to high molecular weight chitosan, low molecular weight chitosan exhibit better antimicrobial activity especially for bacterial pathogens [37] and extensively used for pharmaceutical products [38] .
4. Conclusion
In conclusion, the maximum yield of mycelial chitosan was obtained from A. Butleri NCIM 977 under the cultivation condition of pH 5.5 and temperature 30˚C with production batch time of 72 hrs. Subsequently, the culture media supplemented with glucose, yeast extract and tryptone produced the highest yield of mycelial chitosan. An increase in the yield of mycelial chitosan was dependent on the supplementation and interaction of nitrogen source with other media components. The results indicated that the choice of media and nitrogen supplements are critical factors in obtaining high yield of mycelial chitosan. The media composition also affected the glucosamine content, degree of deacetylation, viscosity and molecular weight of the mycelial chitosan and was found to be 80.68%, 79.89%, 73.22 ml/g and 8.07 × 104 Da respectively. Interestingly, low molecular weight chitosan with desirable physico-chemical properties would be achieved under submerged fermentation and could be potentially applied to the food, cosmetic, chemical and pharmaceutical industries. In summary, fermentative production of mycelial chitosan can be a good alternative to lessen environmental pollution of strong alkali from traditional chitosan production and provides a new, simple and green technology for production of low-molecular-weight chitosan.
NOTES
*Corresponding author.