Protective Effects of Combined Therapy of Rutin with Silymarin on Experimentally-Induced Diabetic Neuropathy in Rats ()
1. Introduction
Numerous epidemiological studies show that the number of diabetic people is increasing due to population growth, aging, urbanization and high prevalence of obesity and physical inactivity [1] . Diabetes is associated with high risk of microvascular complications such as retinopathy, nephropathy and neuropathy [2] . DN occurs about 15% - 25% in type-1 and 30% - 40% in type-2 diabetic patients, causing disabilities and a high mortality rate [3] . Neuropathic pain is defined as a form of chronic pain that results from damage or abnormal function of central or peripheral nervous system [4] . The patients suffering from neuropathic pain frequently report sensory abnormalities such as burning sensations, hyperalgesia, allodynia and dysesthesia [5] . It can also alter the patient’s quality of life by interfering with emotional well-being [6] .
Experimental studies in diabetic rodent models have demonstrated that DN is the result of a complex network of interrelated vascular [7] , metabolic [8] and neurotrophic defects [9] which culminate in electrophysiological deficits, abnormal sensory perception and progressive damage and loss of unmyelinated and myelinated nerve fibers [10] . Metabolic changes that may be involved in the pathogenesis of DN include polyol pathwayflux, increased oxidative stress via glucose autoxidation and the subsequent formation of advanced glycation end products (AGEs), altered eicosanoid metabolism, activation of nuclear enzyme poly (ADP-ribose) polymerase and decreased antioxidant defense [11] .
Oxidative stress is believed to be a biochemical trigger for sciatic nerve dysfunction and reduced endoneurial blood flow in diabetic rats [12] . In this regards, the potential sources of reactive oxygen species (ROS) including endothelial NADPH oxidase, xanthine oxidase, nitric oxide synthase and mitochondrial respiratory chain inefficiency are more notable [13] . Furthermore, diabetes is linked with reduced activity of GST, GPx, GR, Cu-Zn superoxide dismutase and lower levels of glutathione [14] -[16] . Opposite to this, diabetes causes increase in the lipid peroxidation (LPO) products such as MDA or conjugated dienes in sciatic nerves [17] . Enhanced oxidative stress consecutively activates nuclear factor kappa B (NF-jB), which up-regulates genes such as cytokines, adhesion molecules, endothelin-1 and tissue factor [18] . Although DN is traditionally considered a non-immune disease, accumulating evidence now indicates that immunologic and inflammatory mechanisms play a significant role in its development and progression [19] [20] . TNF-α has a major role in the immune system and it increases in type 1 diabetic patients with DN and also shows independent correlation between interleukins and DN [21] .
Epidemiological studies have shown that many phytonutrients of fruits and vegetables might protect the human body against damage by ROS. The consumption of natural antioxidant phytochemicals was reported to have potential health benefits [22] . Flavonoids are known to have powerful antioxidant activities that could play a protective role in oxidative stress-mediated diseases including diabetes, inflammation, hepatotoxicity and cardiovascular diseases [23] -[26] . Rutin (quercetin-3-rutinosid or vitamin-P) is a flavonol glycoside, which is comprised of the flavonolquercetin and the disaccharide rutinose and it is known to have pharmacological activity such as lowering blood pressure and capillary reinforcement and as an anti-inflammatory [27] [28] . Moreover, rutin has inhibitory effects against membrane lipid peroxidation [23] . Silymarin, an extract from the seeds of the milk thistle plant, Silybummarianum, has been used for centuries against liver diseases. Silymarin is a mixture of seven flavonolignans: 1) silybin-A, 2) silybin-B, 3) isosilybin-A, 4) isosilybin-B, 5) silychristin, 6) silydianin, and 7) isosilychristin and one flavonoid named taxifolin. Experimentally evidenced that silymarin has anti-oxidant, immunomodulatory, anti-fibrotic, anti-proliferative and anti-viral activities although its mechanism of action is incompletely understood till date [29] -[31] . When multiple antioxidants are used in combination, they protect against vulnerability to other agents and synergistically potentiate their antioxidant properties [32] . These synergistically potentiated antioxidant effects of agents contribute to the improvement of cognitive function. Thus it was found worth to experimentally examine the neuroprotective potential of combined therapy of RT and SM supplementation on the diabetic-induced changes in development of behavioral and biochemical deficits in rats.
2. Materials and Methods
2.1. Animals
Wistar male albino rats, roughly the same age of 12 - 13 weeks and weighing 250 - 280 g were received from the Experimental Animal Care Center (College of Pharmacy, King Saud University, Riyadh, Saudi Arabia). They were maintained under controlled conditions of temperature (22˚C ± 1˚C), humidity (50% - 55%) and light (12 h light/dark cycles) and were provided with Purina chow (Grain Silos & Flour Mills Organization, Riyadh, Saudi Arabia) and water ad libitum. All procedures including anesthesia were conducted in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research (NIH Publications No. 80-23; 1996, USA) and the Ethical Guidelines of the Experimental Animal Care Center, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia. The ethical clearance has taken form Experimental Animal Care Center.
2.2. Diabetes Induction
Diabetes was induced in overnight fasted rats by a single intraperitoneal injection of STZ (SIGMA Chemicals, USA) at a dose of 60 mg/kg body weight freshly dissolved in 0.1 mol/L citrate buffer, pH 4.5. Control rats as vehicle received equal volume of citrate buffer. The animals with fasting blood glucose values more than 250 mg/dl after 72 h of STZ injection were considered diabetic and included in the study.
2.3. Study Design
Six normal healthy rats were used in control group (vehicle) and the STZ-induced diabetic rats were randomly divided as, diabetic (STZ), RT (100 mg/kg/day) treated to diabetic rats (RT100 + STZ), SM (60 mg/kg/day) treated to diabetic rats (SM60 + STZ) and RT + SM (50 and 30 mg/kg/day respectively) treated to diabetic rats (RT50 + SM30 + STZ). Vehicle and drug treatment were started three weeks after the diabetes induction and continued for six consecutive weeks by orally (Gavage). Behavioral assessments including mechanical hyperalgesia test, tail flick test and Rot rod treadmill test were under taken before and after treatments. At the end of the experiment, rats were fasted overnight for 12 h and blood samples were obtained by cardiac puncture under ether anesthesia. Animals were then sacrificed by cervical dislocation and sciatic nerve tissues were rapidly removed and dipped in liquid nitrogen for a minute and kept in deep freezer at −80˚C till analysis of TBARS, GSH, SOD, CAT, GST, GR and GPx activities.
2.4. Paw Pressure Withdrawal Test
The method described by Sugimoto et al. [33] , was used with slight modifications. In summary, twenty four hours after the lost day of treatment, paw pressure thresholds were recorded with the paw pressure analgesia meter (MK-20D Analgesic meter, Muromachi KIKAI CO. Ltd., Japan). A constantly increasing pressure stimulus was applied to the dorsal surface of the rat hind paw while the animal was gently restrained under a soft towel to avoid tissue damage, a cutoff of 400 mmHg was used. The pressure was increased until the animal withdrew the paw, squeaked or struggled. One measurement per paw was performed with an interval of longer than 15 minutes between measurements; for each animal, the results for the 2 paws were averaged for use in statistical analysis.
2.5. Tail Flick Test
The method described by Sugimoto et al. [33] , was used with slight modifications. Acute nociception was induced by using a tail flick apparatus (Tail Flick model DS 20 Sorrel Apelex, France). Briefly, each rat placed in a restrainer and the tail flick latency was determined by focusing the intensity controlled beam of light on the distal last 2 cm of the animal’s tail and recording the time taken to remove the tail from the noxious thermal stimulus. For each animal, 2 to 3 recordings were made at an interval of 15 minutes; the mean value was used for statistical analysis.
2.6. Measurement of Motor Coordination (Rota Rod Treadmill Test)
The method described by Kamboj et al. [34] , was used with slight modifications. The Rota-Rod Treadmill for rats and mice (Model MK-670, Muromachi Kikai Co, Ltd., Tokyo, Japan) was used. Animals were initially trained to maintain themselves on the rotating rod for more than 2 min. In test trials, the time (in seconds) that trained rats could stay on the rod, rotating at 20 rpm, with a cut off at 60 min was measured. Rats were placed on the rotating rod for two trials each.
2.7. Serum Analysis for What?
Serum fasting glucose levels were estimated by using the commercially available kit (RANDOX Laboratories Ltd., UK) and insulin levels were measured by using enzyme immunoassay (ELISA) kit (DRG, Germany). The serum level of TNF-α, IL-1β and IL-6 were determined using commercially available ELISA kits (R and D systems, Minneapolis, USA).
2.8. Tissue Homogenate for What Measurements?
Sciatic nerve samples were homogenized in 50 mM phosphate buffered saline (pH 7.4) by using a glass homogenizer (Omni International, Kennesaw, GA, USA). Half of the homogenates were centrifuged at 1000 g for 10 min at 4˚C to separate nuclei and unbroken cells. The pellet was discarded and a portion of supernatant was again centrifuged at 12,000 g for 20 min to obtain post-mitochondrial supernatant. In homogenate, TBARS and GSH levels were estimated. In post-mitochondrial supernatant, SOD, CAT, GST, GR and GPx activities were measured.
2.9. Estimation of TBARS Levels in Sciatic Nerve
A TBARS assay kit (ZeptoMetrix) was used to measure the lipid peroxidation products, malondialdehyde (MDA) equivalents. One hundred microliters of homogenate was mixed with 2.5 ml reaction buffer (provided by the kit) and heated at 95˚C for 60 min. After the mixture had cooled, the absorbance of the supernatant was measured at 532 nm using a spectrophotometer. The lipid peroxidation products are expressed in terms of nmoles MDA/mg protein using molar extinction coefficient of MDA-thiobarbituric chromophore (1.56 × 105/M/cm).
2.10. Estimations GSH Levels in Sciatic Nerve
The GSH levels were measured using the method described by Sedlak and Lindsay [35] . Homogenate was mixed with 0.2 M Tris buffer, pH 8.2 and 0.1 mL of 0.01 M Ellman’s reagent, [5,5’-dithiobis-(2-nitro-benzoic acid)] (DTNB). Each sample tube was centrifuged at 3000 g at room temperature for 15 min. The absorbance of the clear supernatants was measured using spectrophotometer at 412 nm in one centimeter quarts cells.
2.11. Estimations of SOD Activity in Sciatic Nerve
The activity of SOD in sciatic nerve was estimated using the method described by Kono [36] , with the aid of nitrobluetetrazolium as the indicator. Superoxide anions are generated by the oxidation of hydroxylamine hydrochloride. The reduction of nitrobluetetrazolium to blue formazon mediated by superoxide anions was measured 560 nm under aerobic conditions. Addition of superoxide dismutase inhibits the reduction of nitrobluetetrazolium and the extent of inhibition is taken as a measure of enzyme activity. The SOD activity was expressed as units/mg protein.
2.12. Estimation of CAT Activity in Sciatic Nerve
The CAT activity was measured by the method of Aebi [37] , using hydrogen peroxide as substrate in post-mitochondrial supernatant. The hydrogen peroxide decomposition by catalase was monitored on spectrophotometer (LKB-Pharmacia, Mark II, Ireland) by following the decrease in absorbance at 240 nm. The activity of enzyme was expressed as units of decomposed/min/mg proteins by using molar extinction coefficient of H2O2 (71/M/cm).
2.13. Estimations of GST, GR and GPx Activities in Sciatic Nerve
The enzymatic activities of GST, GR and GPx were determined by using ELISA kits (Cayman Inc, USA) in the post mitochondrial supernatant of colon homogenate on ELISA reader by following the manufacturer’s instructions.
2.14. Statistical Analysis
All data were presented as the mean ± Standard Deviation (SD). The data were evaluated by a one-way ANOVA using Graph Pad Prism program and the differences between means were assessed using Student NewmanKeuls. The differences were considered statistically significant at P < 0.05.
3. Results
3.1. Effects on Glucose and Insulin Levels
Mean plasma fasting glucose levels significantly (P < 0.001) increased while insulin levels were decreased in STZ-induced diabetic rats. Treatments with RT and SM to diabetic animals for 6 consecutive weeks showed significant (P < 0.05) decrease in fasting glucose and increase in insulin levels when compared to untreated diabetic rats. However, the combined treatment with RT and SM to diabetic rats showed more (P < 0.01) protective effect on glucose and insulin levels (Figure 1).
3.2. Effect on Mechanical Hyperalgesia
In paw pressure analgesia test, vehicle-treated diabetic rats significantly decreased the paw withdrawal latency (PWL) compared to control animals. The diabetic group of animals treated with RT (100 mg/kg/day) for 6 weeks could not significantly altered the PWL time (s) compared to untreated diabetic rats while SM (60 mg/kg/day) treatment produce significant (P < 0.05) increase in PWL time. The combine treatment of RT and SM (50 and 30 mg/kg/day respectively) to diabetic rats showed marked (P < 0.01) increase in time of PWL compared untreated diabetic animals (Figure 2(a)).
3.3. Effect on Tail Flick Latency (TFL)
A significant decrease in TFL was observed after 9 weeks of STZ-induced diabetic rats compared to control animals. This decreased of TFL time in diabetic rats was significantly (P < 0.01) reversed by SM treatment
 (a)
(a) (b)
(b)
Figure 1. Effect of rutin (RT), silymarin (SM) and their combination on serum glucose and insulin levels of diabetic and non-diabetic animals. Data expressed as mean ± SD (n = 6). One-way ANOVA and StudentNewman-Keuls multiple comparisons test was applied and the significance levels considered as *P < 0.05, **P< 0.01 and ***P < 0.001. a: significantly different from control group (P < 0.05) and b: significantly different from STZ group.
while RT could not show such increase in the TFL time. However, the combined therapy with RT and SM to diabetic rats revealed marked (P < 0.01) increase in TFL compared to untreated diabetic animals (Figure 2(b)).
3.4. Effect on Rota Rod Treadmill Performance
Rota-rod treadmill performance of diabetic and non-diabetic animals is shown in Figure 2(c). The running performance on treadmill was significantly (P < 0.001) decreased in diabetic animals compared to control rats. The distinct treatment of RT and SM to diabetic rats showed significant P < 0.05 and P < 0.01 enhancement in running performance when compared to untreated diabetic animals respectively. The combined treatment of RT and SM to diabetic rats enhanced more significantly (P < 0.001) the running performance on treadmill compared to untreated diabetic animals.
3.5. Effects on Pro-Inflammatory Cytokines
Serum pro-inflammatory markers including TNF-α, IL-1β and IL6 levels were markedly (P < 0.001) increased in diabetic rats as compared to control animals. Treatments with RT and SM significantly (P < 0.05) decreased
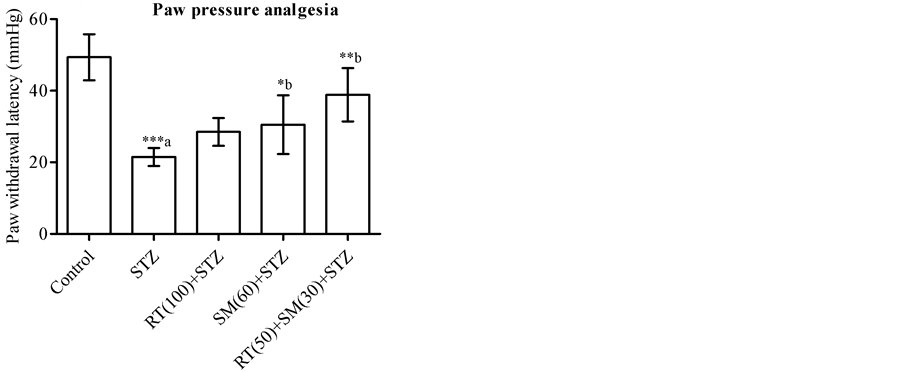 (a)
(a) (b)
(b) (c)
(c)
Figure 2. Effect of rutin (RT), silymarin (SM) and their combination on pain threshold in paw pressure analgesia, tail flick and Rota-rod treadmill performance of diabetic and non-diabetic animals. Data expressed as mean ± SD (n = 6). One-way ANOVA and Student-Newman-Keuls multiple comparisons test was applied and the significance levels considered as *P < 0.05, **P < 0.01 and ***P < 0.001. a: significantly different from control group (P < 0.05) and b: significantly different from STZ group.
the TNF-α and IL-1β values in diabetic rats compared the untreated diabetic animals. The IL-6 levels significantly (P < 0.01) inhibited after SM treatment while the RT treatment did not altered the levels significantly as compared untreated diabetic rats. Comparable to these individual treatment groups the combined therapy of RT and SM showed more significant effect on TNF-α (P < 0.01), IL-1β (P < 0.001) and IL-6 (P < 0.001) levels when compared to untreated diabetic groups respectively (Figure 3).
3.6. Effect on Lipid Peroxidation Bio-Markers
Lipid peroxidation (LPD) bio-markers such as TBARS levels showed significantly (P < 0.001) increased in sciatic nerves of diabetic rats while GSH levels decreased significantly (P < 0.001) compared to control animals respectively. Treatments with RT and SM to diabetic rats for six weeks markedly reduced the TBARS levels and increased the GSH values compared STZ group. However, the combined treatment with RT and SM to diabetic rats showed more significant (P < 0.001) effect on diabetic-induced LPD bio-markers when compared to untreated diabetic rats (Figure 4).
 (a)
(a) (b)
(b) (c)
(c)
Figure 3. Effect of rutin (RT), silymarin (SM) and their combination on serum TNF-α, IL-1β and IL-6 levels of diabetic and non-diabetic rats. Data expressed as mean ± SD (n = 6). One-way ANOVA and Student-Newman-Keuls multiple comparisons test was applied and the significance levels considered as *P < 0.05, **P < 0.01 and ***P < 0.001. a: significantly different from control group (P < 0.05) and b: significantly different from STZ group.
 (a)
(a) (b)
(b)
Figure 4. Effect of rutin (RT), silymarin (SM) and their combination on TBARS and GSH levels in sciatic nerve of diabetic and non-diabetic rats. Data expressed as mean ± SD (n = 6). One-way ANOVA and Student-Newman-Keuls multiple comparisons test was applied and the significance levels considered as *P < 0.05, **P < 0.01 and ***P < 0.001. a: significantly different from control group (P < 0.05) and b: significantly different from STZ group.
3.7. Effects on Enzymatic Activities
The oxidative enzymes including SOD, CAT, GST, GR and GPx activities significantly (P < 0.001) inhibited in diabetic rats compared to control animals. The individual treatment of RT and SM to diabetic animals markedly enhanced these activities compared untreated diabetic animals. The combined therapy of RT and SM to diabetic rats revealed more significant (P < 0.001) effect on inhibited activities of SOD, CAT, GST, GR and GPx than individual supplementation of these compounds (Figure 5).
4. Discussion
Diabetic neuropathic pain is one of the most common diabetic complications that approximately occur in half of the diabetic patients with symptoms of spontaneous pain, allodynia and hyperalgesia [38] . Experimentally induced diabetes by STZ is a well-documented animal model to explore behavioral and pharmacologic changes associated with DN [34] . Thermal sensitivity assessment in diabetic rats and mice was done using a number of behavioral tests, like tail flick, and hot-plate tests. Both thermal hyperand hypo-algesia have been described in STZ-diabetic rats with short-term (2 - 8 weeks) diabetes [19] -[39] . Behavioral methods to test mechanical hyperand hypo-algesia in experimental studies include mechanical withdrawal thresholds in diabetic rodents models assessed by paw (rats) and tail (mouse) pressure Randall-Selitto test or with a von Frey aesthesiometer and rigid von Frey filaments [40] . A decrease of the pressure withdrawal threshold by 30% - 40% after 3 weeks of STZ-diabetes was reported by Romanovsky et al. [41] . Increased nociceptor activity and sensitivity during hyperglycemic hypoxia could be a mechanism or e.g. burning pain attacks in painful neuropathy [42] . In the present study, in diabetic rats, the tail withdrawal latency was significantly shorter than that observed in control animals, indicating development of thermal hyperalgesia. This was accompanied by decreased motor coordination as assessed by performance on Rota-rod treadmill. Most of the phenolic compounds are known to have anti-inflammatory, analgesic and also have anti-nociceptive properties [34] [43] [44] . This may be because of RT and SM combined treatments to diabetic rats for 6 consecutive weeks showed more significant improvement in TFL and PWL rate compared to individual treatment groups. Our findings are agreement with earlier reports that that combined supplementation of phenolic compounds have more potentials than individual treatment [45] [46] .
Hyperglycemia is known to be one of the vital factors that can induce expression of inflammatory biomarkers during diabetes via oxidative stress pathways and associated rise in inflammatory path to induce neural cells death and dysfunction [47] [48] . Present findings obviously show the association between inflammation and the development of DN while the levels of pro-inflammatory biomarkers such as TNF-α, Il-1β and IL-6 were
increased in diabetic animals compared to controls. It is well known that such inflammatory cytokines play a vital role in systemic inflammation and in propagation of acute phase reaction [49] . Indeed, studies suggested that the elevated values inflammatory mediators during diabetes are a consequence of hyperglycemia and insulin resistance [26] [50] . Treatment with RT or/and SM to the diabetic rats significantly reduced these markers whereas the combined therapy produced higher effect. This may be because of RT and SM has antioxidant and anti-inflammatory properties [51] [52] . Kwon and his colleagues, [53] reported that, RT ameliorates dextran sulfate sodium-induced experimental colitis in mice by following attenuation of pro-inflammatory gene expressions. Earlier reports documented that SM has anti-inflammatory and analgesic properties [54] [55] .
Diabetes mellitus induces oxidative stress by the autoxidation of monosaccharides [56] , which leads to production of superoxide and hydroxyl radicals. It is well documented that pain transmission requires production of reactive oxygen species [57] . In present results a significantly higher level of lipid peroxidation marker, MDA, in sciatic nerve of diabetic animals was observed. Glutathione, a potent endogenous antioxidant is a first line of defense against free radicals. The GSH levels were significantly lowered in the sciatic nerve of diabetic animals compared to control group of rats. These observations are in agreement with previous findings showing reduction in GSH levels in diabetes [21] [58] . Intracellular GSH levels have been observed to decrease in brain [34] and sciatic nerve [58] of diabetic animals. The treatments with RT or/and SM significantly reduced lipid peroxidation and regenerated intracellular GSH content in the sciatic nerve; this is probably because of their free radical scavenging activities.
The results of the present study are in agreement with earlier studies wherein decreased SOD and CAT activitieswere observed in nerves isolated from diabetic rats [22] . Reduction in these activities in hyperglycemia might involve non-enzymatic glycosylation [59] . Increased SOD activity after RT or/and SM administration to the diabetic animals is in accordance with reported restoration of SOD activity by rutin in hepatic cells and kindey [52] [60] , and SM in serum [61] and liver [62] . CAT is responsible for the catalytic decomposition of H2O2 to O2 and H2O. The decreased CAT activity in diabetes might reduce protection against free radicals. It is clear that the simultaneous reduction in the activity of both SOD and CAT makes the sciatic nerve more vulnerable to hyperglycemia-induced oxidative stress. Reports are available wherein RT and SM have been shown to bring about improvement in the CAT activity during diabetic-induced nephrotoxicity in rats [52] [63] .
The results obtained emphasize that RT or/and SM protects the sciatic nerve from hyperglycemia induced damage by restoring the activity of both these enzymes. However, the protective results found markedly higher in the combined (RT and SM) group compared to individual groups. GR is an important enzyme involved in maintaining high GSH/GSSG ratios [64] . Present data showed a significant decrease in the activity of GR in sciatic nerve of diabetic animals. The results obtained from the earlier studies also showed depressed GR activity in sciatic nerve [34] and increase and decrease in brain and other organs of diabetic animals [65] [66] . The reversal of GR activity by RT or/and SM treatment might result in increasing intracellular GSH levels. Earlierit has shown that RT and SM protect against the reproductive toxicity effects of cyclophosphamide by increasing GR activity [67] . In present studies, the activity of GPx was found to be significantly depressed in sciatic nerve of diabetic rats and it was reversed by the RT or/and SM treatments. Glutathione reductase is responsible for the regeneration of GSH, whereas GPx and GST work together with GSH in the decomposition of H2O2 or other organic hydroperoxides. A reduction observed in sciatic nerve GR, GPx and GST activity in diabetic rats might be reflection of decreased protein thiols observed in the study as GSH groups play a critical role in enzyme catalysis [68] . The treatments RT or/and SM ameliorates decrease in the activity of these enzymes which might be mediated by GSH regeneration.
5. Conclusion
From the above findings, it has concluded that RT or/and SM have the ability to ameliorate diabetic-induced ND via their anti oxidative and anti-inflammatory properties. The combined therapy of these phenolic compounds is more effective than individual usage particularly in diabetic-induced ND. Thus, combined therapy of RT and SM is recommendable for diabetic patients against neuropathic pain treatment.