The Misleading Use of “Enthalpy” in an Energy Conversion Analysis ()
1. Introduction
In basically all thermodynamic text books the enthalpy H is introduced and used in an energy conversion analysis, predominantly in open thermodynamic systems. For example, Moran and Shapiro ([1] page 81, 128) introduce H in the following way:
In many thermodynamic analyses the sum of the internal energy U and the product of pressure p and volume  appears. Because the sum
appears. Because the sum  occurs so frequently in subsequent discussions, it is convenient to give the combination a name, enthalpy, and a distinct symbol, H. By definition
occurs so frequently in subsequent discussions, it is convenient to give the combination a name, enthalpy, and a distinct symbol, H. By definition  Since
Since  and
and 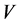 are all properties, this combination is also a property… The appearance of the sum
are all properties, this combination is also a property… The appearance of the sum  in the control volume energy equation is the principal reason for introducing enthalpy previously. It is brought in solely as a convenience.
in the control volume energy equation is the principal reason for introducing enthalpy previously. It is brought in solely as a convenience.
While Cengel ([2] page 52) under the headline “Enthalpy—A combination Property” writes:
In the analysis of certain types of processes, particularly in power generation and refrigeration (...), we frequently encounter the combination of properties . For the sake of simplicity and convenience, this combination is defined as a new property, enthalpy, and given the symbol H.
. For the sake of simplicity and convenience, this combination is defined as a new property, enthalpy, and given the symbol H.
Once a new physical quantity is introduced, one seeks for its physical interpretation. With the enthalpy often it is just the “combination property” explanation as in the two examples given above. Sometimes, however, H is named “heat content” or “total heat” (see [2] for details) without giving a really convincing justification for these names as a general description of what H stands for.
2. Enthalpy: Motivation, Definition, and Interpretation
In the examples given in the introduction the control volume energy equation was mentioned as the principal reason for introducing enthalpy. Before this is explained in more details some general considerations about control volume balance equations and the quantities appearing in these equations are necessary.
2.1. Control Volume Balance Equations
The state of a thermodynamic system is characterized by several physical quantities like mass, energy and entropy which can be subject to a balance with respect to a control volume. In this kind of balance the change of a certain quantity in the system is exclusively due to a transport of this quantity across the control volume boundaries when the quantity under consideration is a conserved quantity (like the thermodynamic total energy). For non-conserved quantities (like entropy) changes may also be due to creation and/or destruction of the quantity.
In these equations two types of quantities appear which are very different in nature:
• state quantities, characterizing a (instantaneous and local) state of the system at a certain time and location within the system. Let i and j be different times or locations then a general state quantity here will be named
 or
or  and changes are denoted by
and changes are denoted by 
• process quantities, characterizing a process by which the state quantities of the system are changed between their states i and j. A general process quantity here will be named 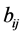 with the double index ij indicating that the process occurs between i and j.
with the double index ij indicating that the process occurs between i and j.
Mathematically, both kinds of thermodynamic quantities are very different: state quantities have a total differential (and thus are path independent), process quantities do not have it.
With n state quantities and m process quantities involved, a general control volume balance equation for a steady thermodynamic system between two cross Sections 1 and 2, for example, reads
 (1)
(1)
Only when balance equations are developed according to this general form a clear physical interpretation can be given.
2.2. The Energy Balance (1. Law of Thermodynamics)
The well-known “1. Law of Thermodynamics” applied to a finite open control volume exemplified for a steady energy conversion is illustrated in Figure 1. The state quantity is the total thermodynamic energy, here introduced as specific energy  which is either
which is either  or
or . It has three parts which are the internal energy
. It has three parts which are the internal energy
 , the kinetic energy
, the kinetic energy 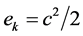 and the gravitational potential energy
and the gravitational potential energy . Process quantities are the work done (
. Process quantities are the work done ( ) and the heat transferred (
) and the heat transferred ( ) with respect to the control volume.
) with respect to the control volume.
According to its general form (1) the energy balance equation reads with 1 for the inlet and 2 for the exit cross section
 (2)
(2)
The process quantities on the r.h.s. of (2) are
• energy transfer across the control volume boundaries between 1 and 2 in form of heat, i.e. due to local temperature gradients, which lead to a local wall heat flux . Then
. Then  with A as transfer area is
with A as transfer area is
 (3)
(3)

Figure 1. State and process quantities involved in a control volume balance for an open system.
• energy transfer across the control volume boundaries between 1 and 2 in form of work, i.e. due to local forces with moving points of action. In an open system there are two work terms which are
 : shaft work, transferred by the shaft of a turbine or a pump
: shaft work, transferred by the shaft of a turbine or a pump
 : flow work, transferred at the inlet and outlet by the action of pressure forces
: flow work, transferred at the inlet and outlet by the action of pressure forces  moving their point of action with the velocity
moving their point of action with the velocity , so that with
, so that with 
 (4)
(4)
The quantities involved in the energy conversion in an open system between 1 and 2 are sketched in Figure 2.
2.3. The Enthalpy-Form of the Energy Balance
When Equation (4) is formally introduced in Equation (2) the energy balance can be written as
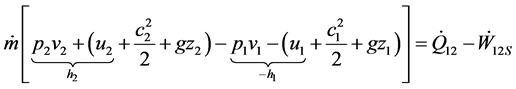 (5)
(5)
Here the specific enthalpy h appears by combining the state quantity u and the process quantity pv. Note that now  is the only work term left since
is the only work term left since  “disappeared” in h. This form of the energy equation is frequently used for open systems, rewritten as
“disappeared” in h. This form of the energy equation is frequently used for open systems, rewritten as
 (6)
(6)
with the specific heat transfer 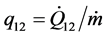 and the specific shaft work
and the specific shaft work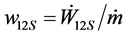 . In this equation
. In this equation 
is often interpreted as an “energy change” of the fluid within an energy conversion process. For example in Warhaft ([3] page 92) it reads with respect to pumps and turbines: They are both devices that change the energy of the fluid by means of work interactions.
In the examples given below it will be shown, however, that combining state and process quantities by using h gives rise to various misinterpretations.
2.4. The Non-Enthalpy-Form of the Energy Balance
The “1. Law of Thermodynamics” balances the total thermodynamic energy which is the sum of a mechanical and a thermal part. Without introducing the enthalpy h it reads in a form corresponding to that of Equation (6) above
 (7)
(7)

Figure 2. Control volume balance for a steady energy conversion in an open system.
Note that now both work terms ( and
and ) appear on the r.h.s. of Equation (7).
) appear on the r.h.s. of Equation (7).
For further analyzing energy conversion systems one can deduce the partial energy equations for the mechanical and the thermal part of the total thermodynamic energy. This is done by multiplying the momentum equation with the velocity in order to get the mechanical partial energy equation. This equation in fluid mechanics is known as Bernoulli equation, see for example Herwig [4] . The thermal partial energy equation then appears when the mechanical equation is subtracted from Equation (7)—guaranteeing that the total thermodynamic energy is the sum of its two parts (mechanical and thermal).
In terms of Equation (7) the two partial equations are:
mechanical: (8)
(8)
thermal: (9)
(9)
Now the specific dissipation  appears which is not explicitly present in Equation (7). The physical background is the following:
appears which is not explicitly present in Equation (7). The physical background is the following:
Dissipation of mechanical energy converts mechanical into internal energy. This energy devaluation process happens, when flow work is not converted into shaft work but increases the internal energy of the fluid. Thermodynamically this corresponds to an entropy generation in this irreversible process. Since, however, flow work  and internal energy increase
and internal energy increase  both appear in the total energy equation, the dissipation term
both appear in the total energy equation, the dissipation term  does not explicitly show up in the total energy equation (6) or (7). Only in equation (8) the specific dissipation
does not explicitly show up in the total energy equation (6) or (7). Only in equation (8) the specific dissipation  appears in a way which is appropriate for the determination of this quantity.
appears in a way which is appropriate for the determination of this quantity.
As mentioned before the specific dissipation is accompanied by an entropy generation 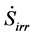 according to
according to
 (10)
(10)
with  as specific entropy generation and T as thermodynamic temperature. Knowing
as specific entropy generation and T as thermodynamic temperature. Knowing  or
or  is essential when losses in a flow field should be determined. For a detailed discussion of this aspect of an energy conversion see Herwig and Schmandt [5] .
is essential when losses in a flow field should be determined. For a detailed discussion of this aspect of an energy conversion see Herwig and Schmandt [5] .
As far as the enthalpy is concerned, h does not only combine a state and a process quantity, but also one that appears in the thermal energy equation (u in Equation (9)) and one that is part of the mechanical energy equation (pv as part of  in Equation (8)). That is why the enthalpy-form (6) of the energy balance cannot be further detailed by determining the mechanical and the thermal parts of it separately. Then, however, the dissipation term
in Equation (8)). That is why the enthalpy-form (6) of the energy balance cannot be further detailed by determining the mechanical and the thermal parts of it separately. Then, however, the dissipation term  cannot be determined within the energy considerations based on the enthalpy concept.
cannot be determined within the energy considerations based on the enthalpy concept.
3. Energy Conversion Examples
Since the introduction of h is motivated by energy conversion considerations four such examples will be analysed based on Equation (6) using h as the crucial “energy quantity” and compared to an analysis avoiding the enthalpy, based on Equations (7)-(9). The interpretation of the results will be named “enthalpy interpretation” and “nonenthalpy interpretation”, respectively.
3.1. Energy Conversion in a Turbine (Dissipation Neglected)
Figure 3 shows a turbine operated with water which may be part of a pumped storage hydro power station. Assuming incompressible fluid ( ), an adiabatic process (
), an adiabatic process ( ), equal cross sections (
), equal cross sections ( ), and equal elevation head (
), and equal elevation head ( ), Equation (6) reduces to (note:
), Equation (6) reduces to (note: )
)

Figure 3. Control volume around the turbine in a pumped storage hydro power station.
 (11)
(11)
or:  (12)
(12)
The enthalpy interpretation is:
Energy of the fluid is converted into shaft work of the turbine.
This, however, contradicts the fact that the energy e of the fluid remains unchanged, because there is no change in
• internal energy, because u for an incompressible fluid is a function of T only and the temperature remains (basically) the same.
• kinetic energy because  holds
holds
• potential energy because  holds.
holds.
From Equation (7) we get
 (13)
(13)
or:  (14)
(14)
The non-enthalpy interpretation is:
Flow work is converted into shaft work of the turbine without changing the energy of the fluid.
3.2. Energy Conversion in a Turbine (Dissipation Accounted for)
In real turbines dissipation occurs, often reducing the available work by the order of 10%.
Based on Equation (6) one gets
 (15)
(15)
which is the same result as before, c.f. Equation (11).
The enthalpy interpretation is:
There is no recognizable effect of dissipation on the shaft work of a turbine.
From Equation (9) we get
 (16)
(16)
so that Equation (7) now becomes:
 (17)
(17)
or: (18)
(18)
The non-enthalpy interpretation is:
There is a recognizable effect of dissipation on the shaft work of a turbine. Shaft work is reduced by dissipation (which increases the internal energy of the fluid).
3.3. Heating the Fluid Ahead of the Turbine (Dissipation Neglected)
The example from section 3.1 is resumed but now with a heat transfer of strength  ahead of the turbine as sketched in Figure 4.
ahead of the turbine as sketched in Figure 4.
From Equation (5) we get for this case
 (19)
(19)
or: (20)
(20)
The enthalpy interpretation is:
From comparing Equation (20) with Equation (12) one might conclude that  increases the shaft work though it is not clear how h behaves under the heat transfer.
increases the shaft work though it is not clear how h behaves under the heat transfer.
From Equation (9) we get (with indices adopted)
 (21)
(21)
so that Equation (7) now becomes:
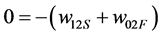 (22)
(22)
or with  since
since 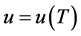 only
only
 (23)
(23)
The non-enthalpy interpretation is:
There is no influence of 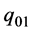 on the shaft work, the internal energy is increased by
on the shaft work, the internal energy is increased by 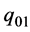 leaving the flow work unchanged.
leaving the flow work unchanged.
3.4. Throttling of an Incompressible Fluid
Figure 5 shows a throttle which may be part of a heat pump or refrigeration cycle and in which an incompressible liquid flows through the throttle. Equation (6) with ,
,  ,
,  and
and  reduces to
reduces to
 (24)
(24)
The enthalpy interpretation is:
The energy of the fluid obviously remains unchanged, “though” dissipation leads to a reduction of the pressure level from  to
to  which is the desired effect.
which is the desired effect.
Equations (8) and (9), however, show that flow work by the irreversible dissipation process (c.f. Equation (10)) increases the internal energy of the fluid, so that (with  and
and ) also the total thermodynamic energy of the fluid is increased.
) also the total thermodynamic energy of the fluid is increased.
The non-enthalpy interpretation is:
The total thermodynamic energy of the fluid is increased by the action of flow work, which leads to a pressure reduction.
4. Conclusion: The Misleading Aspects of Enthalpy
Enthalpy, according to its definition  is a combination of state quantities and thus itself is a state quantity.
is a combination of state quantities and thus itself is a state quantity.
When, however, H or its specific versions h is used in control volume balance equations for an energy conversion analysis only U continues to be a state quantity while pV comes from a process quantity (flow work). Furthermore, the introduction of h in the total energy equation prevents its interpretation as the sum of the partial energy equations for the mechanical and the thermal energy. Then, however, the dissipation term does not appear explicitly (and thus cannot be determined).

Figure 4. Heating the fluid ahead of the turbine.

Figure 5. Control volume around a throttle.
The examples given in Section 3 show several shortcomings of the enthalpy interpretation. Main aspects in this respect are:
• The energy of the fluid and its changes in conversion processes is misinterpreted. Flow work may (as in a throttle) or may not (as in a turbine) change the energy of the fluid. Taking h as an “energy” leads to wrong conclusions.
• The introduction of h combines one term of the thermal energy equation (u in (9)) and one term of the mechanical energy equation (pv in (8)). Then, however, the detailed interpretation in terms of mechanical versus thermal energy is no longer possible.
• The explicit appearance of the dissipation term  only occurs when the partial energies are balanced. This, however, is suppressed by introducing h, so that
only occurs when the partial energies are balanced. This, however, is suppressed by introducing h, so that  is not accessible and thus cannot be determined.
is not accessible and thus cannot be determined.
With all these points in mind the final recommendation is: try to avoid enthalpy in an energy conversion analysis.
Nomenclature
 : general state quantity
: general state quantity
 : area (m2)
: area (m2)
 : general process quantity
: general process quantity
 : velocity (m/s)
: velocity (m/s)
 : specific energy (J/kg)
: specific energy (J/kg)
 : specific internal energy (J/kg)
: specific internal energy (J/kg)
 : specific kinetic energy (J/kg)
: specific kinetic energy (J/kg)
 : specific gravitational potential energy (J/kg)
: specific gravitational potential energy (J/kg)
 : energy (J)
: energy (J)
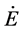 : energyflux (W)
: energyflux (W)
 : specific enthalpy (J/kg)
: specific enthalpy (J/kg)
 : enthalpy (J)
: enthalpy (J)
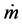 : massflowrate (kg/s)
: massflowrate (kg/s)
 : pressure (N/m2)
: pressure (N/m2)
 : heat flux (W/m2)
: heat flux (W/m2)
 : heat transfer rate (W)
: heat transfer rate (W)
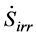 : entropy generation rate (W/K)
: entropy generation rate (W/K)
 : specific entropy generation (J/kgK)
: specific entropy generation (J/kgK)
 : thermodynamic temperature (K)
: thermodynamic temperature (K)
 : specific internal energy (J/kg)
: specific internal energy (J/kg)
 : specific volume (m3/kg)
: specific volume (m3/kg)
 : volume (m3)
: volume (m3)
 : specific shaft work (J/kg)
: specific shaft work (J/kg)
 : specific flow work (J/kg)
: specific flow work (J/kg)
 : work transfer rate (W)
: work transfer rate (W)
 : elevation head (m)
: elevation head (m)
 : specific dissipation (J/kg)
: specific dissipation (J/kg)