Soil Temperature and Phosphorus Supply Interactively Affect Physiological Responses of White Birch to CO2 Elevation ()
1. Introduction
Photosynthetic carbon fixation by trees is the primary contributor to the total productivity of forest ecosystems. A good understanding of how increases in atmospheric CO2 concentration ([CO2]) will affect the photosynthesis of trees will be critical for understanding how climate change will affect the structure, functioning and productivity of forest ecosystems. Photosynthetic responses to CO2 elevations can vary with species, the physiological conditions of plants and environmental conditions. For example, CO2 elevations generally result in a downregulation of photosynthetic capacity in plants that are nutrient-stressed [1-8]. Most studies have shown that nutrient deficiency reduces the benefits of CO2 elevation to plants [2,6,7,9]. However, past studies have generally focused on nitrogen because it generally is the most limiting nutrient element to plants in the field [6,8,10,11]. As another key nutrient element for photosynthesis, phosphorus (P) is often the most limiting or second most limiting element to the aboveground primary productivity of forests [12]. However, the effect of P supply on photosynthetic response to CO2 is not well studied.
P is an essential element for some vital structural and metabolic functions of plants and its deficiency can reduce energy transfer and even lead to a breakdown of cell membranes [13]. P deficiency can limit photosynthesis either indirectly by reducing the total leaf area of a plant [14], or more directly by reducing Rubisco activity and RuBP regeneration [15-18]. Studies using isolated chloroplasts and other in-vitro systems show that P is involved in the activation of Rubisco [19], the modulation of ribulose-5-phosphate kinase and fructose-l,6-bisphosphate phosphatase [20], the transport of triosephosphate (TP) across the chloroplast membrane by the Pi-translocator and the regulation of photophosphorylation [21]. Rao and Terry [22] have found that a deficiency in inorganic phosphate (Pi) results in a substantial increase in non-stomatal limitation to photosynthesis. All of the above physiological processes can affect photosynthetic responses to CO2 elevation. Tissue and Lewis [23] have demonstrated that P deficiency reduces the positive effect of CO2 elevation on photosynthesis in cottonwood.
Soil temperature (Tsoil) also affects plant responses to CO2 elevations. Low Tsoil reduces root permeability and increases water viscosity, leading to a decrease in shoot water potential (Ψ) and stomatal conductance (gs) [5,24, 25]. Reductions in gs and shoot water potential in turn can affect photosynthetic responses to CO2 elevation [5, 25]. For example, low Tsoil is found to reduce the positive effect of CO2 elevations on photosynthesis in various tree species [5,26-29]. Low Tsoil can also reduce the synergistic effect of CO2 elevation and high N supply on photosynthesis [28] and growth [29]. However, the decline in gs at low Tsoil does not necessarily lead to a reduction in photosynthesis in all species [30,31]. Furthermore, low Tsoil has also been observed to reduce the absorption of mineral nutrients directly and/or indirectly by reducing root growth [32-35] or mycorrhizal formation [36]. The availability and absorption of P are particularly sensitive to soil temperature in the boreal forest where it is primarily absorbed through mycorrhizae because of the immobility of the element [25]. However, it is not clear how P supply and soil temperature will interact in affecting tree’s physiological responses to CO2 elevations. As the global warming progresses in response to increasing atmospheric [CO2] and other greenhouse gases, changes in Tsoil will be inevitable because of changes in the depth and duration of snow cover and soil freezing [37]. However, soil warming generally lags behind changes in air temperature. Low Tsoil is prevalent in the boreal forest, particularly at sites with poor drainage and northern and eastern slopes [38,39]. The combination of warmer air temperature and cold soil may severely constrain the response of boreal forests to climate change. Low Tsoil may have been a contributing factor to the wide spread damages to boreal trees by unseasonal warm temperatures [40].
A better understanding of the interactive effects of P supply and Tsoil on the physiological responses of trees to CO2 elevations will be critical for predicting the potential responses of boreal forests to climate changes associated with rising atmospheric [CO2]. This study investigates the interactive effects of P supply and soil temperature on the physiological responses of white birch (Betula papyrifera Marsh) to the doubling of atmospheric CO2 concentration. Since low soil temperature restricts P absorption by roots and P deficiency can lead to photosynthetic down regulation at elevated [CO2], we hypothesize that low Tsoil will result in a greater degree of photosynthetic down-regulation under elevated [CO2], particularly when P supply is low.
2. Materials and Methods
2.1. Plant Materials
The experiment was conducted at the Thunder Bay campus of Lakehead University. White birch seeds were collected from the boreal forest near Thunder Bay and germinated in horticultural trays (28 cm × 56 cm) filled with a mixture of Sphagnum peat moss and horticultural vermiculite (2:1 by volume). This composition has a very high cation exchange, low inherent fertility, a slightly acidic pH (maximizing the availability of all nutrient elements) and a good balance of water holding and aeration porosity [41]. Seedlings of relatively uniform height were transplanted into PVC containers (31.5 cm deep, 11/9.5 cm top/bottom diameter) after 4 weeks of germination and moved to treatment greenhouses as described below.
2.2. Experimental Design
The experiment was a split-split-plot design consisting of two CO2 concentrations (360 ambient vs. 720 elevated µmol∙mol−1, main plot), three Tsoil (split plot) nested within each CO2 treatment (7˚C, 17˚C and 27˚C) and three levels of P supply (split-split plot) nested within each Tsoil (0.1479, 0.3029 and 0.5847 mM P2O5, or 0.2958, 0.6058 and 1.1694 mM P). The soil temperatures and P levels were determined based on the field conditions within the ecological range of the species. Nitrogen and potassium concentrations were 221 and 150 mg/L, respectively, in all treatments. There were two independent replications (greenhouses) for each CO2 treatment, i.e., four separate greenhouses were used. There were eight seedlings per treatment combination. The soil temperature control system consisted of a large box with plant pots mounted and sealed to the bottom and each pot had a 1/2"-diameter drainage hole in the middle. Tsoil was regulated by circulating temperature-controlled water in the space between seedling pots within the large box. The box was insulated to minimize the influence of air temperature on Tsoil. Further details on the soil temperature control system can be found in [42]. The day/night temperatures were 20˚C - 26˚C/15˚C - 18˚C and the photoperiod was 16 hours in all the treatments. All the seedlings were fertilized twice a week with 0.5 L fertilizer solution (which saturated the growing medium). The seedlings were watered frequently to maintain the volumetric water content of the growing medium above 30% as measured with an HH2 moisture meter and a Theta probe (Delta-T Devices Ltd., Cambridge, UK). The temperature of the fertilizer solution and irrigation water was adjusted to the corresponding treatment Tsoil.
White birch is a shade intolerant tree species [43]. It can grow to its full potential under 45% of full sunlight or above. It is very sensitive to moisture, temperature, nutrient and light conditions during the seedling stage. Its seasonal growth can start while daily minimum temperature is below freezing. Seedling height growth can be prolonged indefinitely under long photoperiods [43].
2.3. Gas Exchange Measurements
Six seedlings per treatment combination were randomly selected for measuring photosynthetic response curves to [CO2] after four months of treatment. The measurements were taken using a CIRAS-1 open gas exchange system with an automatic environment control leaf chamber (PP-Systems, Hitchin Hertfordshire, UK) on the first unshaded mature leaf from the top of the seedling (3rd to 5th from the tip). All measurements were taken between 0900 and 1200 hr when gas exchange variables were stable. The environmental conditions in the leaf chamber were as follows: 50% RH, 800 µmol m−2∙s−1 PAR and 26 ˚C leaf temperatures. The A/Ci response curve was measured at 50, 100, 150, 250, 300, 500, 700, 900 and 1500 µmol∙mol−1 CO2. The rate of net photosynthesis at the corresponding growth [CO2] (i.e., 720 and 360 µmol∙mol−1 for the elevated and ambient CO2 treatment, respectively) (Pn) and the rate at 360 µmol∙mol−1 [CO2] for both CO2 treatments (Pn360), transpiration rate and stomatal conductance (gs) were estimated from the response curve of the relevant parameter to measurement [CO2]. Photosynthetic water use efficiency was calculated as IWUE= Pn/transpiration. The rate of in vivo maximal Rubisco carboxylation (Vcmax), rate of photosynthetic electron transport (J), triose-phosphate utilization (TPU) and mesophyll conductance (gm) were calculated using the A/Ci Curve Fitting Utility version 1.1 developed by Sharkey et al. (2007).
2.4. Leaf Nutrient (N, P, K) Assays
Total foliar nitrogen was analyzed using the LECO CNS 2000 dry combustion method [44] and P and K were analyzed using nitric/hydrochloric acid digestion method [45,46]. The nutrient concentrations were expressed on the basis of leaf area and leaf mass. Photosynthetic Nitrogenand P-use efficiencies (hereafter referred to as NUE and PUE, respectively) were calculated by dividing Pn by the corresponding leaf area-based concentration.
2.5. Data Analysis
The Analysis of Variance (ANOVA) were conducted using the Data Desk 6.0 statistical package (Data Description, Ithaca, NY) on the original variables (i.e., no data transformation) since tests showed both ANOVA assumptions (i.e., normality and homogeneity) were satisfied. When a factor with more than two levels (i.e., Tsoil and P) or an interaction was significant, multiple comparisons were conducted using the Least Square Difference (LSD) method. Because of the relatively small sample size in this study and consequently small degree of freedom for the experiment error in the F test (leading to a larger denominator in F calculation), it is more likely for true treatment effects go undetected (Type II error) [47,48]. Therefore we considered a probability above 0.05 but below 0.10 as marginally significant but the interpretation of such results was taken with great precaution. Such a practice is also used in other studies (e.g., [48-50]. However, we have presented the actual probability values in both figures and tables so that the readers can make their own judgement.
3. Results
3.1. Gas Exchange
The CO2 elevation significantly increased the photosynthetic rate (Pn) at the corresponding treatment [CO2] but reduced Pn360 (Table 1, Figure 1(A)). The low Tsoil significantly reduced Pn and Pn360 but there were no significant differences found between the intermediate and high Tsoil at either CO2 treatment (Table 1, Figures 1(A) and (B)).
The CO2 elevation significantly reduced the stomatal conductance (gs) at the intermediate and high but not at the low Tsoil (Table 1, Figure 1(C)). Tsoil had similar effects on gs as it did on Pn and Pn360 under the ambient [CO2] but had no significant effect found on gs under the elevated [CO2] (Table 1, Figures 1(A)-(C)).
The photosynthetic water use efficiency (IWUE) decreased with increasing Tsoil under the ambient [CO2] but was not significantly influenced by Tsoil under the elevated [CO2] (Table 1, Figure 1(D)). The CO2 elevation significantly increased IWUE at the intermediate and high but not at the low Tsoil (Figure 1(D), Table 1).
The soil temperature effect on the mesophyll conductance to CO2 (gm) differed between the two CO2 treat-
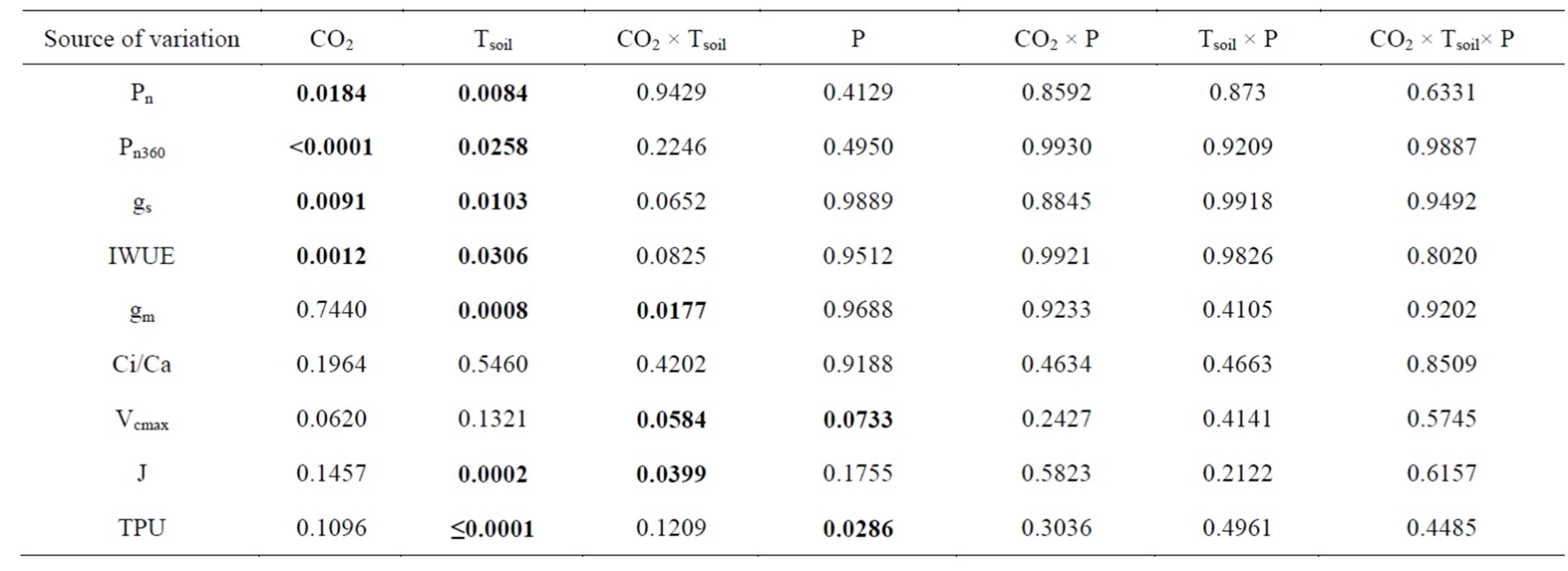
Table 1. Probabilities from ANOVA for the effects of soil temperature (Tsoil), phosphorus supply (P) and [CO2] on net photosynthetic rate at growth [CO2] (Pn), photosynthetic rate measured at a common [CO2] (Pn360), stomatal conductance to water (gs), instantaneous water-use-efficiency (IWUE), mesophyll conductance to CO2 (gm), intercellular/atmospheric [CO2] ratio at the growth [CO2] (Ci/Ca), maximum rate of carboxylation (Vcmax), rate of photosynthetic electron transport (J) and trios-phosphate utilization (TPU) in white birch seedlings. The seedlings were grown at 360 and 720 µmol∙mol−1 [CO2]; 7˚C, 17˚C and 27˚C Tsoil and 241, 493 and 951 mg/L P supply. The soil temperatures and P levels were determined based on the field conditions within the ecological range of the species. Significant probabilities were bold-faced.
ments: at the ambient [CO2], gm was the lowest at the low Tsoil while there were no significant differences between the other two Tsoil; at the elevated [CO2], gm was the lowest at the high Tsoil and highest at the intermediate Tsoil (Figure 2(A)). The CO2 elevation significantly increased gm at the low Tsoil but had no significant effect at other temperatures (Figure 2(A)). The internal to ambient CO2 concentration ratio at the corresponding growth [CO2] (Ci/Ca) was not significantly affected by any of the treatments (Table 1).
3.2. In Vivo Biochemical and Rubisco Activities
Both the maximum rate of Rubisco carboxylation (Vcmax) and the rate of photosynthetic electron transport (J) had the highest value at the intermediate Tsoil while there were not significantly differences between the low and high Tsoil at the ambient [CO2] (Table 1, Figures 2(B) and (C)). At the elevated [CO2], in contrast, Tsoil did not significantly affect Vcmax but J was significantly lower at the high than at the intermediate Tsoil (Figure 2(C)). The CO2 elevation significantly reduced Vcmax only at the intermediate Tsoil and J at both intermediate and high Tsoil (Figures 2(B) and (C)). Furthermore, Vcmax generally increased with increasing P supply but the effect was not statistically significant at 0.05 P level. Although the P effect appeared to be affected by [CO2] and Tsoil, the interactions were not statistically significant (Figure 2(B), Table 1).
The low Tsoil significantly reduced the rate of triose phosphate utilization (TPU) (Table 1, Figure 2(D)). TPU generally increased with increasing P supply but the difference between the low and intermediate P levels was not statistically significant (Figure 2(D)).
3.3. Foliar Nutrient Concentrations and Nutrient Use-Efficiencies
The low Tsoil significantly reduced leaf K concentrations at the ambient but not at the elevated [CO2] (Table 2, Figures 3(A) and (B)). The CO2 elevation significantly reduced foliar K concentrations at the intermediate and high but not at the low Tsoil (Figures 3(A) and (B)). Mass based foliar K concentration increased with increasing P supply (Figure 3(A)).
The low Tsoil significantly reduced both mass based (Pm) and leaf area based P concentration (Pa) under the ambient [CO2] but not under the elevated [CO2] (Table 2, Figures 3(C) and (D)). The CO2 elevation significantly reduced both Pm and Pa at the intermediate and high Tsoil but not at the low Tsoil (Figures 3(C) and (D)). As with Km, Pm at all three Tsoil and Pa at the intermediate and high Tsoil increased with increasing P supply (Figures 3(C) and (D)); The low Tsoil decreased Pm at all three P levels (Figure 3(C)) while it reduced Pa only in the high P treatment (Figure 3(D)).
Nm generally increased with increasing P supply under the ambient [CO2] and high Tsoil while there was no clear trend in other treatment combinations (Table 2, Figure 3(E)). The CO2 elevation generally decreased Nm with some minor variations with P and Tsoil (no clear patterns, Figure 3(E)). The effects of Tsoil also varied with [CO2] and P levels but did not show clear general patterns except the low Tsoil reduced Nm at the low P level under elevated [CO2] (Figure 3(E)). Na was the highest at low P and lowest at the high P under the low Tsoil but
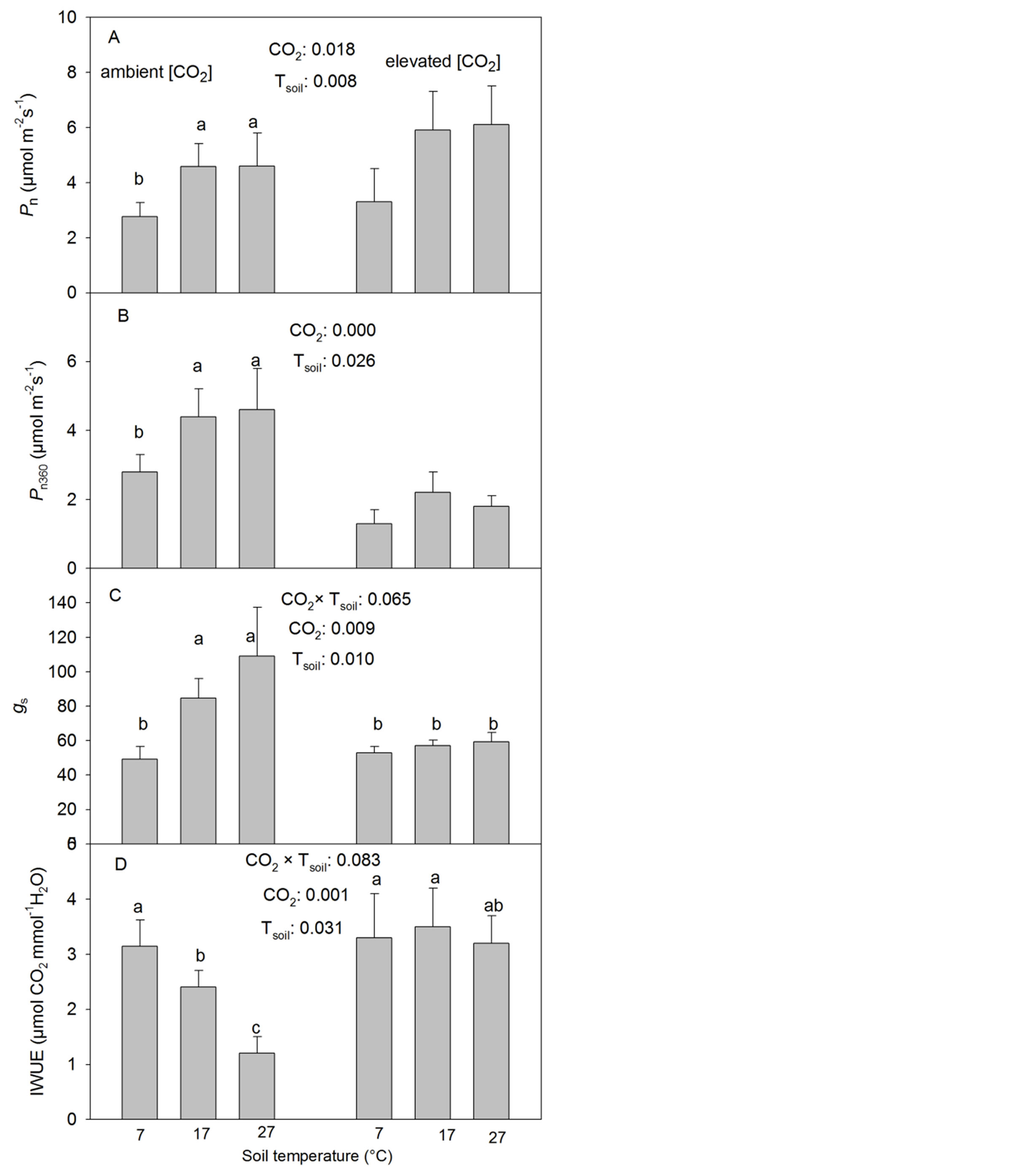
Figure 1. Effects of CO2 concentration and soil temperature (Tsoil) on the rate of net photosynthesis at growth CO2 (Pn) and the rate measured at a common ambient CO2 concentration (Pn360), stomatal conductance to water (gs) and instantaneous water-use-efficiency (IWUE) (mean + SE, n = 6) in white birch seedlings. The seedlings were grown under two [CO2] (360 and 720 µmol∙mol−1), three Tsoil (7˚C, 17˚C and 27˚C) and 3 levels of P supply (0.1479, 0.3029 and 0.5847 mM P2O5). Means with the same letter(s) over them are not significantly different from one another (p > 0.10). Only significant treatments were labeled. Tsoil effects are labeled only on the side of the ambient [CO2] since there were no significant interactions between CO2 and Tsoil (p > 0.10). ***: p ≤ 0.01; **: 0.01 < p ≤ 0.05; *: 0.05 < p ≤ 0.10.

Figure 2. Effects of CO2, Tsoil and P on mesophyll conductance to CO2 (gm), maximum rate of carboxylation (Vcmax), rate of photosynthetic electron transport (J) and rate of triose-phosphate utilization (TPU) in white birch seedlings. Means with the same letter(s) or number(s) are not significantly different from each other (p > 0.10). Other explanations are as in Figure 1.
the trend was the opposite at other Tsoil Table 2, Figure 3(F)). The CO2 elevation significantly reduced Na (Table 2, Figure 3(F)).
The CO2 elevation significantly increased both photosynthetic phosphorus use-efficiency (PUE) and photosynthetic nitrogen use-efficiency (NUE) (Table 2, Figures 3(G) and (H)). The low Tsoil significantly reduced


Figure 3. Effects of CO2, Tsoil and P on foliar nutrient concentrations and photosynthetic N and P use efficiency in white birch seedlings. Non-significant effects were pooled in Figures B, G and H to provide a clearer presentation of significant effects. In Figure D, upper case letters indicate Tsoil × P interactions while lower case letters are for CO2 × Tsoil interactions. Other explanations are as in Figures 1 and 2.

Table 2. Probabilities from ANOVA for the effects of Tsoil, P supply and [CO2] on mass-based leaf potassium concentration (Km), area-based leaf potassium concentration (Ka), mass-based leaf phosphorus concentration (Pm), area-based leaf phosphorus concentration (Pa), mass-based leaf nitrogen concentration (Nm), area-based leaf nitrogen concentration (Na), photosynthetic phosphorus use-efficiency (PUE), nitrogen use efficiency (NUE) and total leaf area per seedling in white birch. Other explanations are as in Table 1.
the NUE (Table 2, Figure 3(H)).
4. Discussion
Both the CO2 elevation and low soil temperature led to photosynthetic down-regulation in white birch but the physiological mechanisms of the down-regulation differed. Photosynthetic measurements at 360 µmol∙mol−1 CO2 suggest that both the low Tsoil and CO2 elevation caused a down-regulation of photosynthesis, but the physiological processes/traits associated with the down regulation were different between the two treatments. While the declines in stomatal conductance contributed to photosynthetic down regulation in both treatments, weakness in sink strength for the utilization of photosynthates (as indicated by the rate of triose phosphate utilization,) was only a contributing factor in the low soil temperature treatment. The contributions of mesophyll conductance and biochemical and photochemical capacity to photosynthetic down regulation were complicated by the interactions between CO2 and soil temperature. For example, the low Tsoil-induced decline in Pn360 was associated with declines in mesophyll conductance under the ambient [CO2], but not under the elevated [CO2]. The CO2 elevation resulted in significant declines in the maximum rate of Rubisco carboxylation, photosynthetic electronic transport and mesophyll conduction in some but not all soil temperatures.
The effects of low Tsoil on foliar gas exchange are complicated, involving interactions between belowground and above-ground parts of the plant [5,24,25]. Larger sample sizes and more comprehensive diagnostic measurements including the measurement of additional characteristics (such as chlorophyll fluorescence parameters, analyses of water relations) may be necessary to discern the physiological mechanisms governing the changes in photosynthesis in response to the above two situations. Regardless of the mechanisms, the photosynthetic acclimation to elevated CO2 was partial since the photosynthetic rates measured under the corresponding growth CO2 concentration were significantly greater under the elevated than under the ambient CO2 concentration. A complete acclimation would have resulted in a photosynthetic rate at the elevated [CO2] being equal to that at the ambient [CO2].
The conclusions of photosynthetic down regulation varied with the specific parameters used in this study. Photosynthetic down regulation is assessed using several different variables in the literature. The maximum rate of Rubisco carboxylation (Vcmax) and maximum rate of photosynthetic electron transport (J) are often used to indicate changes in the biochemical capacity and photochemical capacity of the photosynthetic machinery [2,5, 7,51,52]. Although there was a decline in both parameters associated with the low soil temperature under the ambient CO2 in this study, it was not the case under elevated CO2; furthermore, there was no decline in Vcmax or J in response to the CO2 elevation. Pn360 is often used as an indicator of the integrated acclimation of photosynthetic capacity and stomatal conductance [8,52,53]. To a certain degree, differences in Pn360 reflect a shift in both the supply function and demand function of photosynthesis [25]. However, as discussed previously, the changes in Pn360 in this study primarily reflect changes in the supply function, i.e., the effects of stomatal conductance. These results suggest that Pn360 can be used to indicate the magnitude of photosynthetic down regulation but offers no clue to the mechanisms responsible for the down regulation unless it is considered in conjunction with other parameters. Moreover, Pn360 will not be of any use for predicting photosynthetic performance under a future climate with a doubled atmospheric CO2 concentration because plants will not likely experience the present atmospheric CO2 concentration in the future once the CO2 concentration in the atmosphere is doubled.
The data suggest that white birch will likely have higher water use efficiency (WUE) in the future under elevated atmospheric CO2 concentration, particularly under warmer soil temperatures. The significant increase in photosynthetic rate and simultaneous decline in stomatal conductance both contributed to the increase in water use efficiency under the elevated CO2. However, such changes did not occur at the low soil temperature. In fact, the water use efficiency declined with increasing soil temperature at the ambient CO2 concentration. At the elevated CO2, in contrast, WUE at warmer soil temperatures (17˚C and 27˚C) was as high as that at the low soil temperature (7˚C). These results suggest that in the future the photosynthesis of white birch can be increased by warmer soil temperatures without compromising water use efficiency, which occurred under the ambient CO2 concentration. While the soil temperature will most likely increase as the global air temperature increases, further increases in soil temperature can be achieved through silvicultural means, such as site preparation [54] and manipulation of canopy coverage [55] and soil moisture [38]. The lack of significant responses in stomatal conductance to the CO2 elevation under the adverse soil temperature (i.e., 7˚C) could be interpreted as that the stomatal conductance at the low soil temperature was already at such a low level that it could not go down any further. However, it is also possible that the low soil temperature reduced root growth and thus water intake and transport, which indirectly affected the stomatal conductance and photosynthesis through its impact on leaf water relations [56].
Our results demonstrate that the tradeoff between water use efficiency and nutrient use efficiency depends on the driving factor or factors that cause changes in their use efficiencies. Generally there is a tradeoff between water use efficiency and nutrient use efficiency [25]. Such a tradeoff exists because within the normal operating range of internal CO2 concentration an increase in stomatal conductance will lead to a linear increase in transpiration rate but a curvilinear (thus smaller) increase in photosynthesis, resulting in a decrease in water use efficiency (ratio of photosynthesis to transpiration). On the other hand, any increase in photosynthesis will result in an increase in nutrient use efficiency since nutrient concentrations in leaves are constant over a short period of time [25]. Physiological acclimations will complicate the issue. In this study, the low soil temperature increased water use efficiency and decreased nitrogen use efficiency under the ambient CO2 concentration. Under the elevated CO2, in contrast, the low soil temperature decreased nitrogen use efficiency without a corresponding increase in water use efficiency. Furthermore, the CO2 elevation increased both nitrogen use efficiency and water use efficiency.
The low soil temperature and low P supply did not affect the CO2 elevation induced photosynthetic down regulation in white birch. The hypothesis that the degree of photosynthetic down-regulation in response to CO2 elevation would be greater under low Tsoil and low P supply was based on the argument that the low Tsoil would suppress the uptake of phosphorus and the resulting lower P concentration in the foliage would in turn exacerbate the photosynthetic down regulation induced by CO2 elevation because of the low availability of inorganic phosphorus for the Kelvin Cycle of photosynthesis [25]. However, the basis for the hypothesis did not hold in this study. While there was indeed a significant interaction between soil temperature and phosphorus supply on leaf area based P concentration, soil temperature did not significantly affect foliar P in the low P treatment. Furthermore, P supply did not significantly affect foliage P in the low soil temperature treatment. Therefore, it is not surprising that the indicators for photosynthetic down regulation did not vary in the way proposed in the hypothesis.
Our results suggest that white birch seedlings responded to low P supplies morphologically by reducing the size and total number of leaves rather than through physiological adjustment. No significant physiological response to low P supply was detected in this current study. However, we have previously found that the low P supply led to significantly smaller-sized leaves and smaller amount of leaves per tree [57], indicating a different strategy that white birch used in coping with low P supply than some other tree species. For instance, Tissue and Lewis [23] have reported that low P supplies diminish the positive effect of CO2 elevation on light saturated rate of photosynthesis. We offer two explanations for the different responses between the two studies. Firstly, it is possible that different species respond differently. Plants can respond to P deficits physiologically, morphologically or both [25]. Maybe the prevalent form of response varies with species. Secondly, the range of P supply was different between the two studies. While the P level in the high P treatment of [23] was comparable to ours (0.50 vs. 0.58 mM P2O5), their low P supply was much lower than ours (0.004 vs. 0.15 mM P2O5). Therefore, it is possible that the low P level in this study was not low enough to trigger physiological responses and that morphological adjustment is the first line of responses to low P supplies in white birch.
This study has shown some complicated interactions among soil temperature, nutrient supply and ratios of different nutrient elements. For instance, the CO2 elevation reduced Ka and Pa only at the intermediate and high soil temperature while it resulted in lower Na at all three soil temperatures. Increasing phosphorus supply increased Km but the response of Na showed different patterns at different soil temperatures. These results demonstrate the complicated nature of interactions among nutrient absorption, allocation, physiological functions and their impact on physiological responses to CO2 elevations under different nutrient regimes. Interactions among several factors are much more difficult to study than the main effects of one or two factors and that probably explains the lack of such data in the scientific literature. However, our data show that such interactions in the real world situations can throw the results of studies with simple designs out of context or relevance under certain circumstances. Of course studies involving one or two treatment factors are very important for understanding the mechanisms of their effects. However, the interactive effects of multiple factors are probably more important for making more realistic predictions of plant responses and therefore warrant more attention in future research. Furthermore, interactions involving more than two factors are difficult to visualize and present. There is an urgent need to develop new techniques or to adopt techniques from other disciplinary areas for analyzing and presenting the results of studies with multiple treatments. Common or standardized expressions of interactive effects should facilitate the comparison of different studies and the utilization of results in further efforts such as modeling and predicting whole plant or ecosystem responses to climate changes.
Acknowledgements
We appreciate the technical support of Joan Lee, the Greenhouse Manager of Lakehead University, during the course of the experiment.
Funding
This research was supported by National Science and Engineering Research Council, Canada Foundation for Innovation and Ontario Innovation Trusts grants to Q.L. Dang and Lakehead University Graduate Assistantship to G. Danyagri.
NOTES