Pb concentrations and isotopic compositions in the soil and sediments around the abandoned mine in southwest of Korea ()
Keywords: Pb Isotope Ratios; MC-ICP-MS; Abandoned Mine; Stream Sediment
1. INTRODUCTION
Pb contamination in mine areas followed by ore digging and smelting has been reported through various environmental samples such as mine waste water, soils and plants [1-6]. Heavy metal pollutants released from mines are known to keep affecting the neighboring areas not only during the mine operation but even after the mines became disused. For example, the Pb concentrations of surface soils near the inactive mine in southwest of Spain were found 100 times as high as those of natural background [4]. Surface sediments of streams near the abandoned mine in Scotland were also reported to have a Pb concentration level similar to that of mine wastes [1]. All these indicate that the release of Pb pollutants from mines continues to have impact on the environment even after finishing the mine operation.
Over the past few decades, Pb pollution in mine areas has been evaluated based on concentrations and crustal enrichment factors. However, the total concentrations are not sufficient for a precise evaluation of contamination sources especially in the case of multiple sources for contaminants. Pb isotope ratios have thus been introduced as effective tracer for environmental pollution. Each source of Pb has its own isotopic ratio range. The isotopic composition of Pb in environmental samples reflects the mixing of these sources, and source apportionment can be quantified in cases where all potential sources of Pb are characterized [7]. For example, the surface soils in the Swiss National Park are influenced from natural Pb, Pb ores in mines and air pollutants, and that the relative contributions of these three sources are 43%, 21% and 36%, respectively [8].
In Korea, about 900 abandoned mines are known to be distributed around the nation. Since 1992, the Korean government has investigated environmental impact in the abandoned mine areas. The investigation showed that more than 90% of these areas suffered from serious soil contamination by heavy metal pollutants [9]. According to [10], the soils around Imcheon mine, which has been disused since the 1970s, have an average Pb concentration of 410 mg/kg, which reaches 40% of the maximum allow levels of heavy metals in factory and industrial areas provided by the Soil Conservation Act of Korea. Also, the Pb concentrations of soils near Duckum mine, which has been stopped its operation since 1994, are also found up to 200 times as high as those of natural soil [11]. However, these studies mostly focus on monitoring pollutants, and thus they do not effectively present contributions of pollutants necessary for compensation or reparation when environmental disputes arise. In this study, we investigated Pb isotopic compositions as well as Pb concentrations of soils and sediments around an abandoned mine. This provided the first application of Pb isotope ratios in the Korean abandoned mine area to identify the source for Pb pollutant and assess the relative contribution from that source.
2. MATERIAL AND METHODS
2.1. Sample Collection and Acid Digestion
For the research, surface sediment and soil samples were collected around an abandoned mine located in the southwest province of Korea. The alluvial system of this area consists of many tributary streams from surrounding mountains with one stream from the entrance of the abandoned mine (Figure 1). For the comparison of Pb isotope ratios between Pb contaminant from the aban-
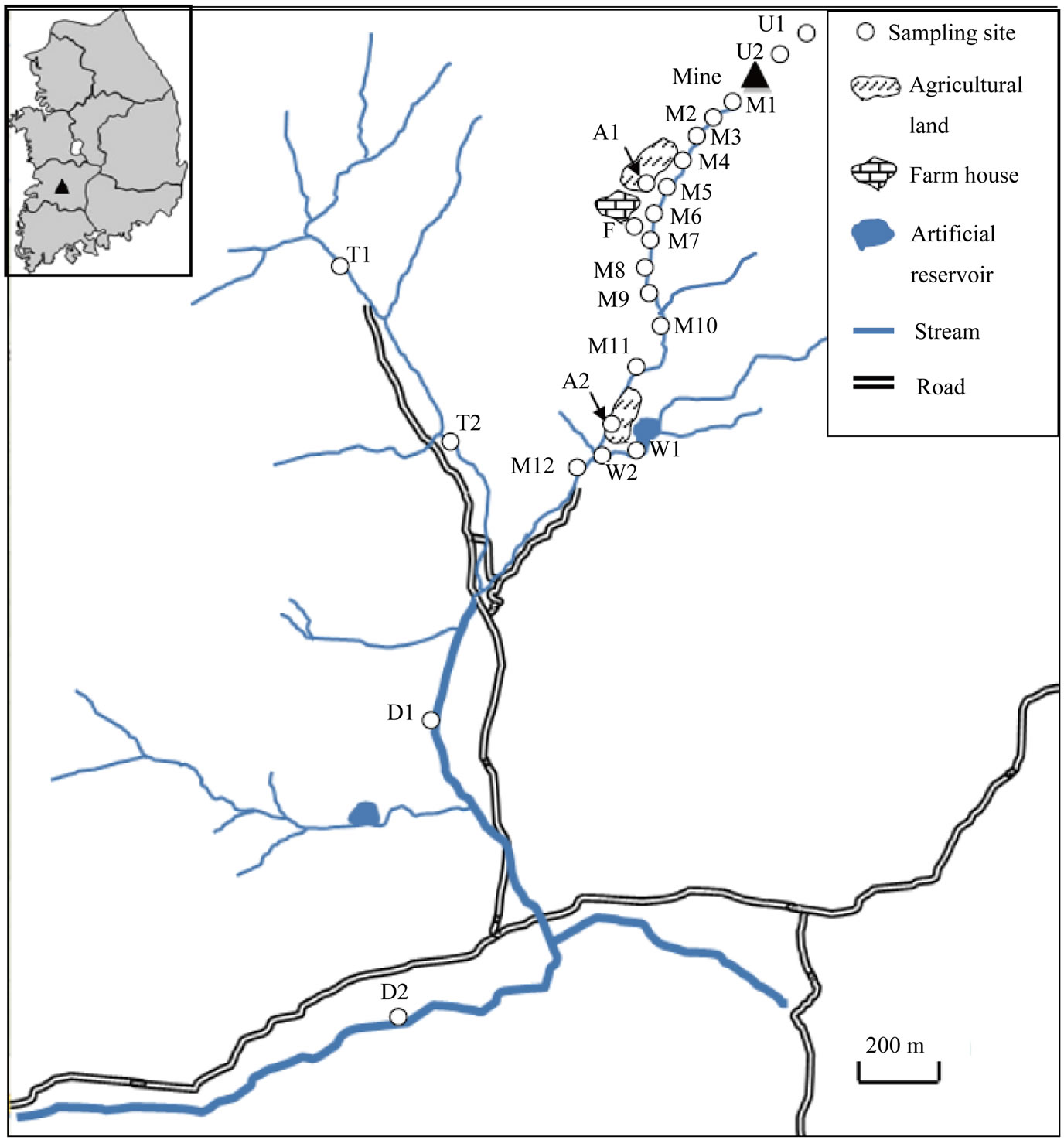
Figure 1. Location of sampling sites near the abandoned mine in southwest of Korea. M1 - M12 are the main stream sediments; U1 and U2 are the mountain soils upper than the mine entrance; A1 and A2 are the agricultural soils; F is the sediments of a creek from the farm house; W1 and W2 are the sediments of the creek from the artificial reservoir; T1 and T2 are the tributary sediments; and D1 and D2 are the sediments of the downstream area.
doned mine and natural Pb, we collected twelve sediment samples at the main stream from the mine entrance (M1 ~ M12), two samples at the tributary stream coming from other direction (T1, T2) and two samples at the downstream area which goes down past the confluence (D1, D2). In addition, seven sediment and soil samples were collected from mountainside upper than the mine entrance (U1, U2), creeks flowed from a farm house (F) and artificial water reservoir (W1, W2) and agricultural fields nearby the main stream (A1, A2) to investigate the influences from potential contaminant sources.
Each sediment sample was dried at room temperature in the class 1000 clean booth for one week. The dried samples were sieved with non-metallic sieves and acid-digested. The acid digestion carried out in this study followed the procedure modified from the EPA method 3050, 3051 and 3052. Approximately 200 mg of each sample powder was loaded into an acid-cleaned Teflon vessel with 5.0 mL of the mixture of HNO3 and HClO4 (4:1 v/v), and then dissolved at 175˚C for 12 hours. The digested samples were dried down on a graphite heating block at 175˚C, reconstituted in 5.0 mL of the mixture of HF and HClO4 (3:1 v/v), and re-dissolved at 175˚C for 12 hours. The samples were then dried again and sequentially re-dissolved by 1.0 mL of HCl and 5.0 mL of the mixture of HClO4 and H3BO3 (1:3 v/v). Finally, the samples were diluted with 10 mL of 1% HNO3 and then centrifuged at 3000 rpm for 10 minutes. About 8 mL of supernatant was collected from each sample for the measurement of Pb concentrations and isotope ratios.
2.2. Chemical Separation
Since MC-ICP-MS performance can be affected by various spectral interferences, a targeted element should be separated from the sample matrix [12]. The Pb in each sample was separated through anion exchange column separation. The column purification was done as below: 2 mL of Biorad® AG-MP1 resin was packed in Savillex Teflon micro column and cleaned using HNO3, HCl and HF [13]. Before column separation, the resin was conditioned with 10 mL of 7 N HCl and 0.001% H2O2 solution mixture; and 1 mL aliquot of each digested sample was dried and redissolved in 1 mL of that solution. Then, it was loaded into a column. The elution was done in the following sequence: 10 mL of 7 N HCl + 0.001 % H2O2 (for Pb and the matrix), 24 mL of 7 N HCl + 0.001 % H2O2 (for Cu), 12 mL of 1 M HCl (for Fe), 8 mL of 0.01 M HCl (for Zn), 8 mL of HCl (for the matrix) and 10 mL of 0.001 M HCl (for Cd). Because the modified procedure aimed to perform multi-separation of Pb, Cu, Fe, Zn and Cd through one stage, Pb and the matrix were separated together from other elements such as Cu, Fe, Zn and Cd at the first elution. In order to separate Pb from the sample matrix, second elution was done using 4 mL of 1 N HCl (for the matrix) and 8 mL of 0.1 N HCl (for Pb).
2.3. Analysis of Pb Concentrations and Isotope Ratios
Pb concentrations and isotope ratios were analyzed with the ICP-OES (Optima 5300 DV, Perkin Elmer) and the MC-ICP-MS (Nu Plasma II, Nu), respectively, at the National Institute of Environmental Research (NIER). The DSN-100 desolvating system including a micromist nebulizer was used for sample introduction of the MCICP-MS. Typical instrumental parameters of the MCICP-MS are presented in Table 1. The mass bias during the analysis of Pb isotope ratios was corrected using NIST SRM 997 Tl spiked in samples. In addition, the Pb isotope ratios of NIST SRM 981 Pb spiked with NIST SRM 997 Tl were measured after every five samples to check the instrumental drift. The 206Pb/207Pb and 208Pb/207Pb ratios of NIST SRM 981 determined in this research were 1.0932 ± 0.0001 and 2.3688 ± 0.0003, respectively. These values well agreed with the previous research [14]. The variations in Pb isotope ratios of NIST SRM 981 were less than 130 ppm, and thus the influence from the instrumental drift was not corrected.

Table 1. Instrument operating conditions of the MC-ICP-MS.
3. RESULTS AND DISCUSSION
3.1. Pb Concentrations of Stream Sediments
The Pb concentrations and isotopic compositions of the sediment and soil samples are summarized in Table 2. The Pb concentration data clearly showed the enrichment of Pb in the main stream sediments compared with the other sites (Figure 2). The Pb concentrations determined in the main stream sediments ranged from 139.0 - 1079.43 mg∙kg−1. These values were similar to the Pb concentrations in the soils and sediments at various mine impacted areas in Korea [10,11,15]. On the other hand, the soil and sediment samples collected at the other sites


Table 2. The concentrations and isotopic compositions determined in soil and sediment samples. The uncertainties of data (95% significance level) are 13.5% for Pb concentrations, 0.1% for 208Pb/207Pb and 0.2% for 206Pb/207Pb based on duplicate measurements of M9 sample.
showed relatively low Pb concentrations (18.56 - 29.52 mg∙kg−1) similar to the mean concentration of Pb in the upper continental crust (17 mg∙kg−1) [16]. Since the agricultural soil near to the mine entrance (A1) showed low Pb concentrations, it can be inferred that the Pb pollutant from the mine was transported only through the main stream, and thus atmospheric pollutants deposition and/ or dispersal of soluble Pb was negligible in this area. Among the other sites, D1 displayed slightly increased concentration, and this might result from confluence of the main stream.
3.2. Spatial Distribution of Pb Isotope Ratios
The most striking features in Pb isotopic compositions were high 208Pb/207Pb ratios and low 206Pb/207Pb ratios of the main stream sediments compared with the other sites (Table 2, Figure 2). The 208Pb/207Pb ratios of the main stream sediments ranged from 2.4854 to 2.4883, and only three sites (D2, W1 and W2) among the other sites showed higher 208Pb/207Pb ratios than those of the main stream (Table 2, Figure 2). According to [17], the higher abundance in 208Pb was the characteristic feature of Korean Pb ores. Thus high 208Pb/207Pb ratios observed in the main stream sediments suggest the influence of Pb from the closed mine over these sites. The 206Pb/207Pb ratios of the main stream sediment ranged from 1.1693 to 1.1740 were the lowest values determined in this research. Unfortunately, Korean Pb ore are known to have a large variation in 206Pb/207Pb ratios [17-19]. Therefore it is difficult to characterize 206Pb/207Pb ratios of Pb ore. However, the 206Pb/207Pb ratios of the main stream sediment samples quite well met those of Pb ores in several mines located in the southwest province of Korea (Figure 3). In addition, the 206Pb/207Pb ratios of the marine sediment in the Southern East Sea decreased during 1930~1990 indicating low 206Pb/207Pb ratios of anthropogenic Pb in Korea [19]. Therefore it can be inferred that low 206Pb/207Pb ratios of main stream sediments represent the contributions of Pb from the closed mine to these sites.
The Pb isotope ratios of sediments collected from the other sites such as tributary stream, the downstream area and the creeks showed large variations depending on sites (Figure 2). Since no significant pollutions were found in corresponding sites, the differences between the sites might result from speciation of natural Pb after deposition. In this research, since all samples were total digested using HF, it was impossible to investigate the speciation of Pb in these sites.
3.3. Source Identification and Binary Mixing System
Figure 3 shows a three-isotope plot of 206Pb/207Pb

Figure 2. Pb concentrations and isotope ratios determined in sediment and soil samples. Each error bar represents uncertainty based on the duplicate measurements of M9 sample.. The solid lines and dashed lines represent the mean values and 2 σ values of whole Pb isotope ratios of the main stream sediments, respectively.
versus 208Pb/207Pb ratios for all soil and sediment samples. The Pb isotopic compositions of the main stream sediments (M1 - M12) were close to the isotopic signatures of the Pb ores in the mines located in the southwest of Korea [18], while the Pb isotope ratios of the mountain soils upper than the mine entrance (U1 and U2) were clearly different from those of the main stream (Figure 3). This indicates that the Pb in the main stream sediments mainly originated from the closed mine.
In Figure 3, the Pb isotope ratios of the main stream sediments (M1 - M12), the tributary stream sediments (T1 and T2) and the downstream sediment sample (D1) display good linear correlation (R2 = 0.9185, p < 0.001). In addition, the isotopic composition of D1 is plotted between those of the main stream and the tributary stream sediments. This suggests that the isotopic composition of the Pb in the downstream area resulted from mixing of the main stream and tributary stream. As mentioned above, the Pb in the main stream sediments dominantly came from the closed mine. On the other hand, the Pb in the tributary stream sediments were probably natural Pb, because there were no pollution sources around the tributary stream. The low concentrations of Pb at T1 and T2 similar to the mean concentration of global continental crust also support little contribution of anthropogenic Pb in the tributary stream. Therefore, it can be inferred that the Pb in the downstream area were the mixture of anthropogenic Pb from
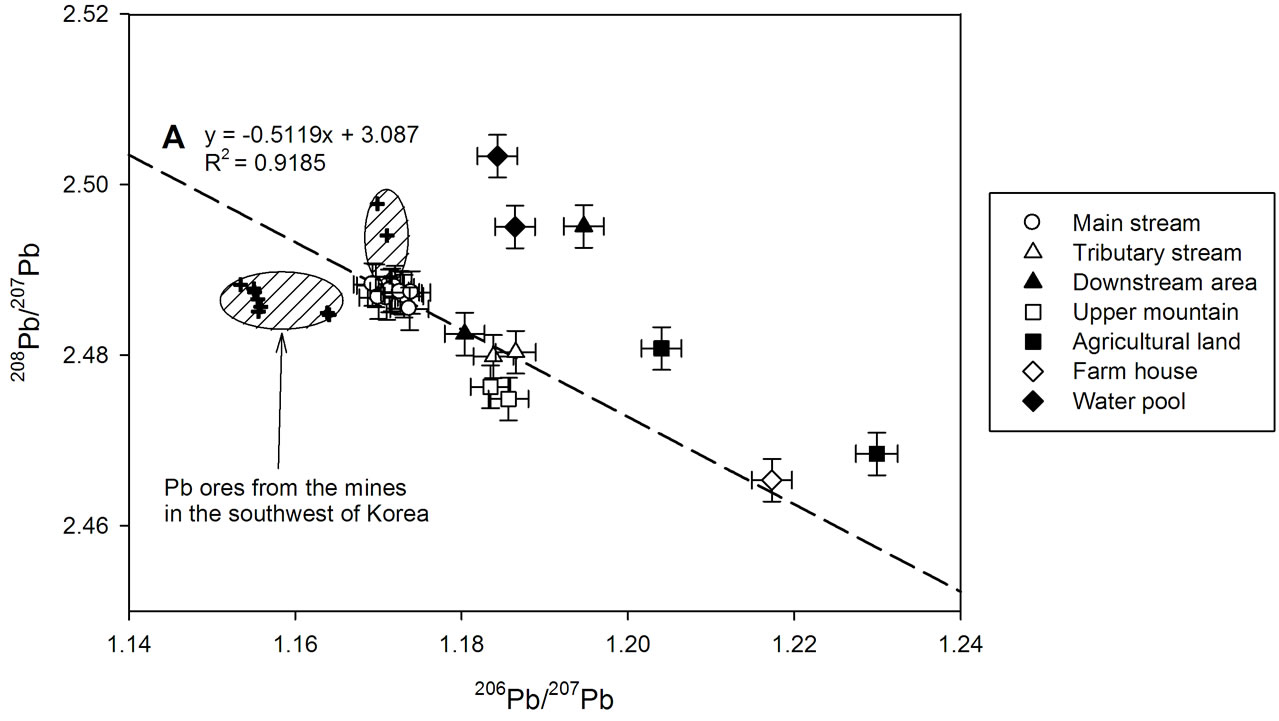
Figure 3. The three-isotope plot for 206Pb/207Pb vs. 208Pb/207Pb in soil and sediment samples. The dashed line (A) represents the linear regression of Pb isotopic composition of the main stream sediments (M1-M12), the tributary stream sediments (T1-T2) and one of the downstream area (D1), while the dash-dotted line (B) represents that of artificial water pool, (W1, W2), one of the agricultural soil (A2) and sewage from the farmhouse (F). The crosses in the shaded area were the Pb isotope ratios of Pb ores in several mines located in southwest of Korea [18].
the closed mine and natural Pb from the tributary stream.
According to [19], if samples were mixtures of two component end members such as natural and anthropogenic Pb, linear trend can be obtained from the isotope ratio and the inverse of concentration. In this research, high linear correlation (R2 = 0.9492, p < 0.001) between M1 ~ M12, T1 ~ T2 and D1 was also observed in the isotope ratio versus inverse of concentration plot (Figure 4). In two end member mixing system, the relative contribution from each end member can be calculated by a simple binary mixing equation as below [20]:
 (1)
(1)
where FM and FT represent the relative contributions of anthropogenic Pb from the closed mine and natural Pb from the tributary stream, respectively; (208Pb/207Pb)M, T, D are the isotope ratio of each end member and mixture sample (D1). Since there were no additional sources, the boundary condition, FM + FT = 1, can be applied, and the equation (1) can be solved as below:
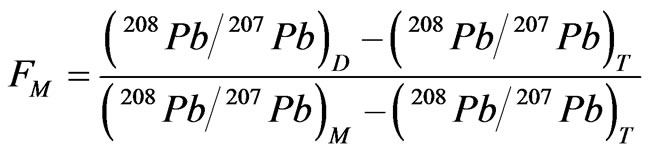 (2)
(2)
According to the above calculation with the mean 206Pb/207Pb and 208Pb/207Pb ratios, the relative contributions of the abandoned mine to the downstream area (D1) are about 36 and 33%, respectively. In our knowledge, this is the first assessment of a relative contributions from a closed mine in Korea. Thus, no data are available to compare with. In addition, the influence from a closed mine can be changed according to weather conditions and/or geochemical characteristics of a region [21]. However, the similar results calculated with different isotope ratios may represent authenticity of our assessment.
4. CONCLUSION
The high concentrations of Pb up to 1080 mg∙kg−1 in the main stream sediments represent the contamination by the Pb pollutants from the closed mine. It is also supported by the Pb isotope ratios of the main stream sediments which are similar to Pb ores of mines in southwest of Korea. On the other hand, the influence of the closed mine is not appeared in the tributary stream and other creeks around the main stream. The Pb isotopic compositions of the stream sediment samples also suggest a twosource mixing system between the main stream, the tributary stream and the downstream area. The relative contribution of Pb from the mine to the downstream area reaches up to 36%. However, it can be changed according to the weather condition. Conclusively, this study reveals that the Pb isotope ratios are useful tracer not only for identification of pollutant source but also for assessment of its relative contribution.
5. ACKNOWLEDGEMENTS
The results presented in this paper were obtained in the framework of the project “Improvement in Precision and Accuracy of Isotope

Figure 4. Inverse concentration plot versus 208Pb/207Pb ratios of the main stream, the tributary stream and downstream area sediment samples. The dashed line represents the linear regression between these sites except D2.
Analysis of Heavy Metals by MC-ICP-MS” which is financially supported by the National Institute of Environmental Research.