Hematology and blood serum chemistry reference intervals for children in Iganga district of Uganda ()
1. INTRODUCTION
Complete blood count (CBC) [1] and serum biochemistry test [2,3] are commonly used not only to assess overall health but also to diagnose and monitor disease as well as determine the effect and safety of interventions including treatment and vaccines. Increasingly, many clinical trials in Africa involve children and pregnant women [4-6]. However, although considerable efforts have been expended into developing capacity in the conduct of clinical trials in many sites across Africa towards improved intervention against diseases [7,8], information on the normal hematologic and serum biochemical features of African children is scanty. By and large, the normal ranges of blood cell counts and serum biochemical constituents currently used in Africa are derived from data collected from populations living in industrialized countries of North America and Europe. Many of these imported hematology and blood biochemistry values are available in textbooks or in the guidelines provided by manufacturers of laboratory test kits and equipment. Yet, such reference intervals for European populations are not identical to the normal values for populations in the disease endemic trial sites [9]. For example, adult Africans have been reported to have lower levels of hemoglobin, red blood cells, platelets, neutrophils, eosinophils and monocyte counts compared with populations in the Western countries [10-13]. Similarly, hematology and immunology tests of Eastern and Southern African adult populations show differences between the African and the European reference values. Judged against standard ranges recommended by World Health Organization [10, 14], blood specimens of Africans were found to have lower hemoglobin, hematocrit, red blood cell count (RBC), mean corpuscular volume (MCV) and neutronphils and platelets counts, while levels of monocytes and eosinophils were higher.
Based on such differences in hematology and blood serum biochemistry normal ranges of African versus European and North American adult populations, it is clear that the use of “imported” reference values generated from distant or dissimilar populations may misguide the interpretation of blood cell counts and serum biochemistry findings, causing not only misdiagnosis but also misreporting of adverse events. Whereas normal ranges of blood cell count and serum components for adults differ from those of children [15], information sources of hematology and serum biochemistry normal ranges for African children are scarce [16]. Therefore, in the present study, normal reference ranges of hematology and blood serum biochemistry for healthy Ugandan children aged 12 to 60 months have been determined. Uganda hosts one of three clinical trial sites (the others are in Burkina Faso, Ghana and Gabon) that are carrying out phase 2b investigation of blood-stage P. falciparum malaria vaccine GMZ2 [17]. The participants for the present study are recruited from households in six villages constituting a malaria-study cohort within a newly established health demographic study site (HDSS) [18,19] in Iganga district where extensive clinical trials are anticipated. It is hoped that because of shared anthropologic and socio-economic backgrounds across Africa, the determined reference ranges will be widely applicable to children in sub-Saharan and other regions of Africa [16].
2. MATERIALS AND METHODS
2.1. Study Population
Between November and December of 2008, we conducted a population based cross-sectional survey to establish the normal ranges of hematology and serum biochemistry values for children resident in six villages (Nakavule, Busei B, Busei South, Bulubandi, Nandekula, Kasokoso) of Iganga-Mayuge Health and Demographic Surveillance Site of Makerere University (HDSS), Uganda which has been described recently [18-20]. This project was part of a baseline study designed to describe malaria indicators in HDSS as well as to prepare a children’s cohort for malaria vaccine clinical trials. Majority of the inhabitants in HDSS are of Basoga ethnic group who contribute at least 10% of the population of Uganda and whose customs and socio-economic conditions are generally similar to those of other Bantu tribal groups in Uganda [21]. Using a stratified random sampling of households, we enrolled one child aged between 12 months to 60 months for every selected household. If a household had more than one eligible child, a ballot method was used to select the trial participant from that household. All children received a physical examination and review of medical history by interviewing parents or guardians using a standardized questionnaire. Only apparently healthy children examined by the study physician and lacking any clinical indication of organic illness were enrolled; any febrile child, children reportedly afflicted with chronic illnesses (including sickle cell disease and diabetes) and those who were on any medication were excluded. Inclusion criteria were: age range 12 to 60 months, parental/guardian written informed consent, willingness to donate a blood specimen, and reported anticipated residence within the study area for at least one year. The informed consent documents were available in English and the local language, Lusoga. All selected households were located within a radius of about 10 km from the study clinic which is situated at Iganga District Hospital. All demographic and clinical information for each participant were recorded in the study questionnaire.
2.2. Hematology and Serum Biochemistry
Blood was collected in the morning hours (between 9:00 a.m. and 1:00 p.m.) by venipuncture under aseptic conditions, distributed in 2 ml tubes (vacutainerTM, Becton Dickinson, Franklin Lakes, NJ): one tube containing ethylene diamine-tetracetic acid (EDTA, purple top) and the other, a plain tube (vacutainer, red top tube). The blood was then transported at ambient temperature to a central laboratory within 3 hours of drawing. Hematological analysis, including complete blood count (CBC) [1] was performed on the EDTA anti-coagulated blood using the Coulter ACT5 diff apparatus (Beckman Coulter, Fullerton, California). The complete blood count included the following parameters: hemoglobin (Hb), red blood cell count (RBC), hematocrit (Hct), mean corpuscular volume (MCV), platelets, while blood cell (WBC) and a differential blood test. To obtain serum, the nonanticoagulated blood (in red top tubes) was centrifuged at 800 g for 15 min. The serum was transferred to 2 ml microtubes and tested using the Roche Cobas Integra 400 plus autoanalyzer (Roche, Indianapolis, Indiana). Serum biochemistry analysis comprised of tests for 14 constituents including albumin, alkaline phosphatase, aminotransferases, globulins, total protein, electrolytes, enzymes, bilirubin, urea and creatinine. Both the hematology and serum biochemistry tests were performed according to the manufacturer’s instructions. In addition, for each child, a stained blood smear was examined to detect Plasmodium or other blood parasite infections.
2.3. Quality Control
Known standards, internal and external controls were run in parallel with specimen tests to assure satisfactory accuracy and precision. External controls were run in cooperation with Clinical Pathology Unit of Mulago Referral Hospital and the Ebenezer Clinical Laboratory, Uganda (certified by Sanas, South Africa: www.sanas.co.za). All clinical, laboratory and data personnel involved in the study undertook training in good clinical and laboratory practices
2.4. Ethics
Study protocols were approved by the Science and Ethics Committee of the College of Health Sciences, Makerere University (approval number 2008-069) and by the Uganda National Council for Science and Technology (approval number HS 765). All children found to be infected with malaria parasites were treated with artemether-lumefantrine [22] in accord with Uganda’s national policy.
2.5. Data Handling and Analysis
Data management was carried out using Microsoft Office AccessTM 2003 and Microsoft Excel 2003 while statistical analysys was performed using Stata V. 12.0 (Stata Corp, College Station, Texas, USA). The mean, median, 5th and 95th percentiles were determined for each tested parameter. Normal reference ranges, p values for differences, and standard deviation from means were calculated. Two-sided p values of less than 0.05 were assumed to show statistical significance.
3. RESULTS
3.1. Study Population
A total of 1168 children resident in Makerere University founded Iganga-Mayuge Health and Demographic Study Site (HDSS) were screened for baseline studies towards subsequent evaluation to participate in a malaria vaccine trial (Figure 1). Six hundred children (58%) were excluded due to either history of fever in the previous 24 hours or presence of asexual malaria parasites in their peripheral blood or existing fever (tympanic temperature ≥ 37.5˚C). Four hundred and sixty children (39.4%) were enrolled in the baseline study (Table 1). The majority (96.7%) of the participants were of Basoga ethnic background while the rest constituted other tribes. Of the 460 healthy enrolled children, thirty (6.5%) were excluded from data analysis due to missing information. In addition, the volumes of about 228 serum specimens
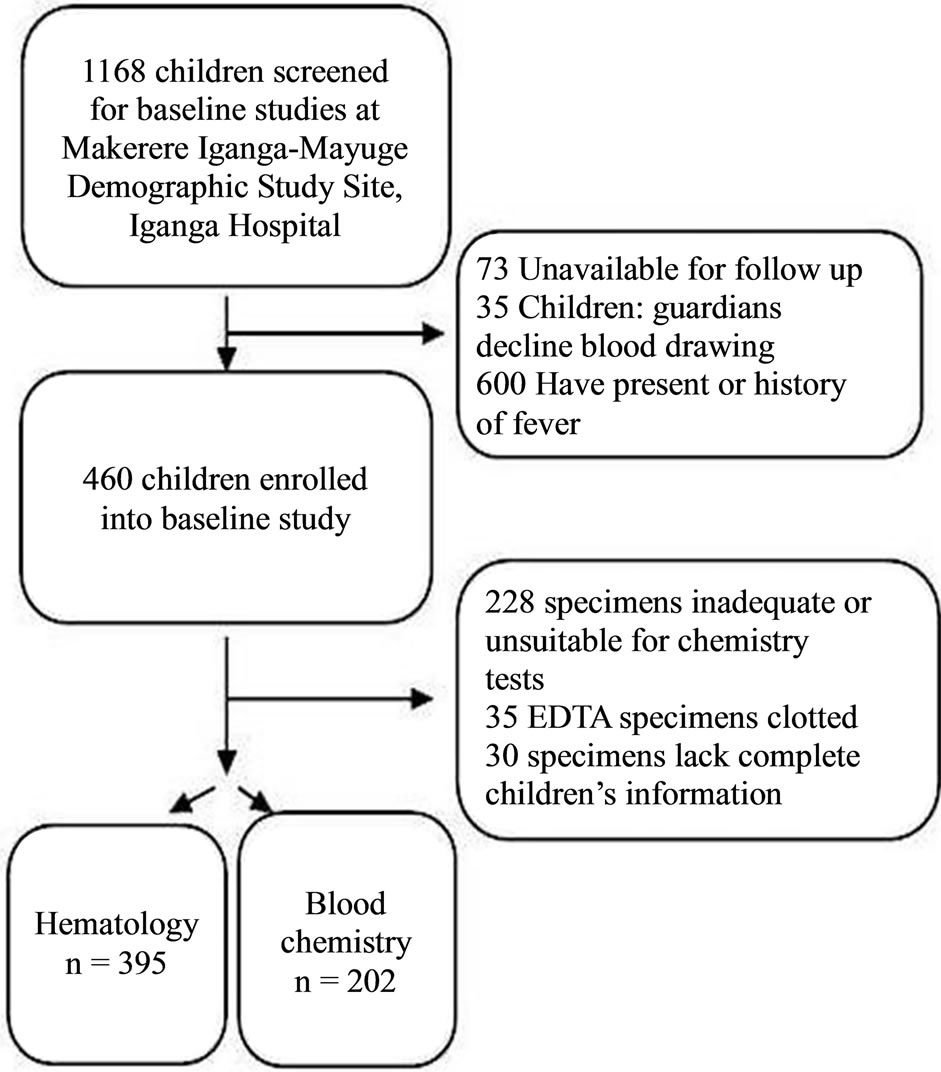
Figure 1. Study profile. To determine local physiological ranges, children resident in Iganga, Uganda were recruited for hematology and blood chemistry.
were inadequate or unsuitable for biochemistry analysis, while EDTA supplemented blood specimens of 5 children were clotted and could not be analyzed for hematology. With these exclusions, 395 specimens (85.6%) for hematology and 202 (43.9%) for biochemistry were included in the analysis for determining the physiological reference ranges. The number of subjects tested for each analyte was within the recommended standard sample size (N = 120) as suggested by CLSI [23]. Gender of the recruited children was evenly balanced, with females constituting 49.8%; the mean age was 3.2 years (SD: ±1.2); and median age was 3 years.
3.2. Hematology
Hematologic reference values and their respective intervals for 20 parameters commonly used in clinical medicine were established for the Ugandan children (Table 1). There were no statistically significant differences in hematology values between male and female children (p > 0.05). It is notable that the lower limits of hemoglobin, hematocrit levels, mean corpuscular volume (MCV) and platelet counts for the Ugandan children (Table 2) are all lower than conventional reference values [24]. On the other hand, the white blood cell count (WBC × 10−3) reference values (median: 8.9, interval: 5.9 - 14.3) for Iganga children are higher compared to the Caucasian normal reference value (interval 4.0 - 11.0) [24].
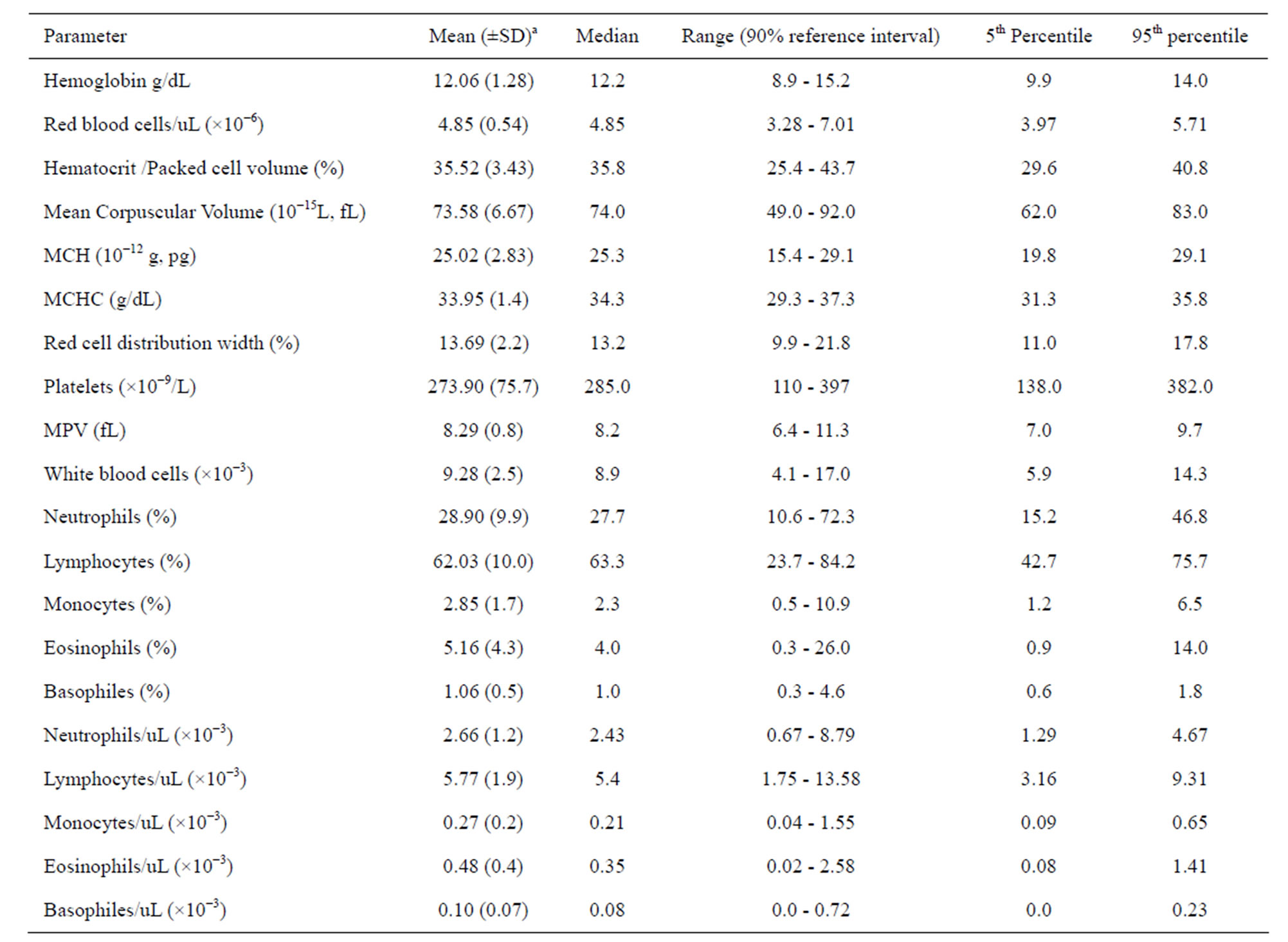
Table 1. Medians, 5th and 95th percentiles for hematology values of children (N = 395, age: 1 to 5 years) from villages of Iganga demographic study site in Uganda.
a. Hematology values for the children (n = 395) were normally distributed.
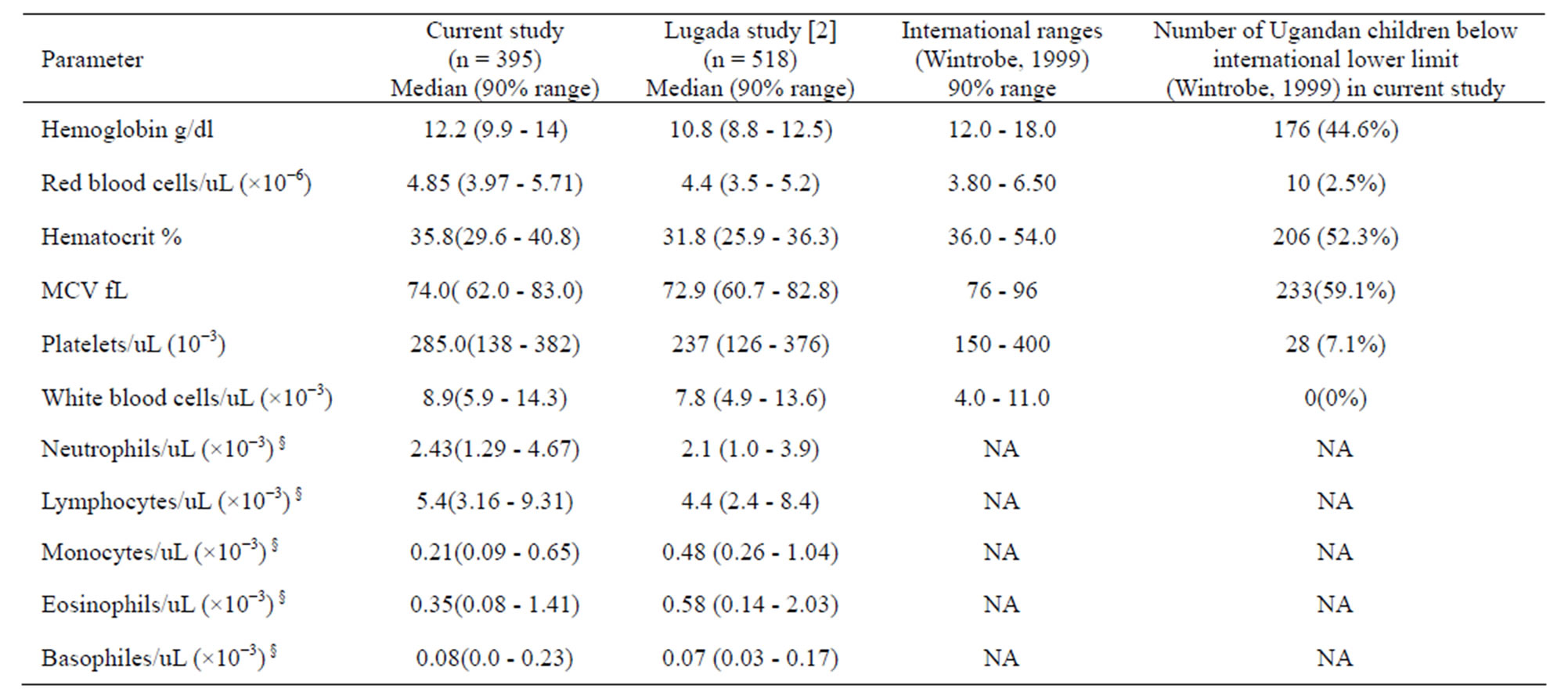
Table 2. Comparisons of medians; 90% reference ranges of hematological indices of Ugandan children and international values.
NA: not applicable. §: indicated range values refer to absolute total specific cells per microliter.
3.3. Serum Biochemistry
Biochemistry reference intervals for the Ugandan children were established for 14 analytes including electrolytes, liver function tests, and renal function tests. Mean and median values as well as the 5% - 95% ranges for 14 analytes are presented in Table 3, along with the corresponding reference intervals for a predominantly Caucasian pediatric population [25]. It is noticeable that the upper limits of serum transaminases, bilirubin, creatinine, urea, total protein and albumin for the Ugandan children are higher than those reported for the Caucasian pediatric population [25]. These differences between biochemical interval limits for the Ugandan and Caucasian children are worth noting since both liver and kidney test profiles are important in monitoring the safety of trial interventions. There were no statistically significant differences between biochemistry values of male and female children (p > 0.05).
4. DISCUSSION AND CONCLUSIONS
Hematology [24] and serum biochemistry reference intervals that are commonly used for African children are derived from studies conducted in Caucasian populations of industrialized countries. However, that CBC and serum biochemistry reference intervals of Caucasian populations differ from the normal ranges that African people have widely reported [10-12,14,26] and are highlighted in a recent report from Gabon [16]. Here, we present reference values for a healthy pediatric population aged 1 - 5 years in HDSS, Iganga, which is an emerging clinical trials site in Uganda. To the best of our knowledge, this is the first paper to describe serum biochemistry reference ranges of children aged 1 to 5 years in Uganda and is one of very few such reports [12,13,16] in Africa. The present results are useful not only for diagnosis and treatment but also enrolling, follow-up evaluation and care of children in clinical trials, including for accurate detection of adverse events in Africa.
This study confirms that, whereas Ugandan children have hematology parameter medians that are comparable with the corresponding values of the reference ranges for Caucasian populations, the minimum normal values of Hb, hematocrit, MCV and platelets for the Ugandan children are lower than the cognate minimum levels for Caucasians (Table 2). In particular, the 5th percentiles for hemoglobin, hematocrit, and Mean Corpuscular Volume ranges for Ugandan children (Tables 1 and 2) are much lower than for populations of the industrialized countries [24]. This implies that based on red cell parameters (Table 3, see column 5), as many as 40% to 50% of the Ugandan children would be classified as unsuitable for recruitment to clinical trials or as adverse events during the trial. Lower hematology parameters in otherwise healthy Africans have been previously reported for adults and children [10,14,27]. This disparity
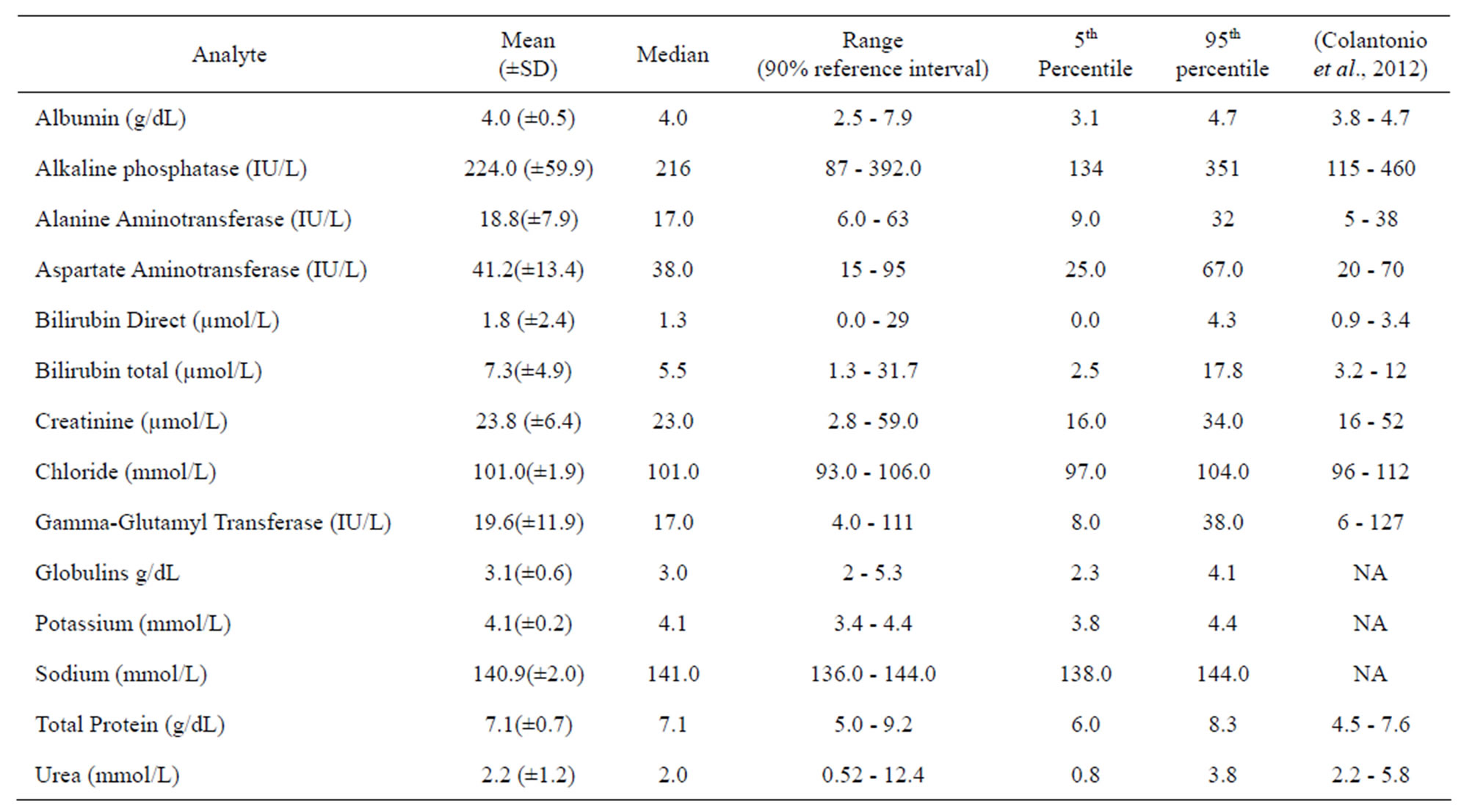
Table 3. Medians, 5th and 95th percentile reference intervals for clinical chemistry values in Ugandan children aged 1 - 5 years.
NA: not available in the report of (Colantonio et al., 2012) but largely within normal range of Caucasian populations (Hay, Hayward, Levin, & Sondheimer, 2001).
may be attributed to several factors including iron-deficiency, chronic blood loss due to hookworm infestation, chronic or recurrent malaria or undiagnosed hemoglobinnopathies. Prevalence of hemoglobinopathies is not yet well established for the Ugandan population.
It is notable that while hemoglobin, hematocrit, and absolute red blood cell count for children recruited in this study and a previous study from Uganda [10] is generally comparable, the red blood cell parameters for our study population are slightly higher compared with those of Lugada et al. [10]. This difference may be due to variance between the two geographic settings. The present study is carried out at HDSS, Iganga, which is a hub of clinical trials that have concurrently enjoyed several health related interventions [28] such as mass anti-helminthes de-worming, insecticide Treated Net (ITN) distribution, health education and mass immunization campaigns.
In conclusion, hematology and serum biochemistry normal ranges for Ugandan children differ from the normal reference intervals of Caucasian children. The lower limits of normal values of Hb, hematocrit, MCV and platelets for the Ugandan children were less than the corresponding minimum levels for Caucasians. On the other hand, the upper limits of serum transaminases, bilirubin, creatinine, urea, total protein and albumin for the Ugandan children were higher than those traditionally used for the Caucasian children. Since CBC and serum biochemistry normal ranges are important for diagnosis and monitoring, establishing and use of local reference ranges should enhance patient care and health research. Clearly, more such studies of children in Africa are desirable, as they will extend the present findings, towards establishing normal reference intervals of local populations for improved care and conduct of clinical trials.
Study Limitations
While physical examinations were carried out, and anthropometric measures were collected for some children, this information was not used during the analysis of hematology or biochemistry results. Thus, it might be possible that some children had mild illnesses, growth stunting, or some degree of malnutrition. Underweight and stunting, in particular, have been shown to be associated with reduced hemoglobin and higher WBC counts in children in Africa [29]. It is also not possible to screen for all of the medical conditions that would have influenced hematological and biochemistry parameters. Such ailments may include helminthes infection, hepatictis, micronutrient deficiency, or hemoglobinopathy. However, the fact that we include a relatively large number of apparently healthy children in our study makes it likely that our data are representative of healthy Ugandan children from the local population.
5. ACKNOWLEDGEMENTS
We are very grateful to Professor Wen L. Kilama for his assistance and advice in the concept and accomplishment of this work. We thank the staff from Malaria Program of Makerere University College of Health Sciences: Ronald Naitala, Henry Wannume, Diana Businge, Patrick Monami, Aisha Nansubuga and Esther Nakawungu for technical support. We are grateful to the Iganga cohort children, parents and guardians for agreeing to participate in the study. This study was conducted with the grant support of African Malaria Network Trust (AMA NET), EuropeAid Co-operation Office, (AIDCO), The European & Developing Countries Clinical Trials Partnership (EDCTP) and Makerere University College of Health Sciences (MakCHS).
