Congruence Study between the Continuous Chemical Reactor (CSTR) and the Maturation Oxidation Ponds within Wastewater Treatment ()
1. Introduction
The bio-rotor system, also known as a rotating biological contactor (Masoud et al., 2005), trickling filters (Masoud et al., 2012), integrated duckweed and Maturation Oxidation Ponds or water stabilization ponds (WSPs) system (Machdar et al., 1997; Ali et al., 2020), activated sludge (Mitikka-Eklund et al., 1999; Ali, 2011), dissolved air flotation (Strous et al., 1999; Masoud et al., 2002), reed bed systems (Rim et al., 1997), all are wastewater treatment techniques. Maturation Oxidation or stabilization ponds are created in a simple scientific manner with 2.4 - 6.3 feet depth, where Biological Oxygen demand (BOD) reduction from wastewater occurs by developing the sustenance of bacterial-algal subsistence life (Ahmed et al., 2019). Ponds for Oxidation and Maturation are a man-made shallow basin that uses herbal techniques beneath in part-managed conditions to reduce natural dependency and harmful organisms annihilation in waste waters (Aboulfotoh, 2017). Ponds for oxidation and Maturation are defined as a shallow, artificial basic that uses natural processes under partially regulated settings to eliminate harmful organisms and decrease organic and inorganic materials in effluent of domestic sewage in several locations (El-Deen Ali Mohamed et al., 2004).
The oxidation pond is a useful, simple, and low-cost method for purifying wastewater before it is released into an aquatic media (Mahajan et al., 2009). The pond of oxidation has attempted to be one of the most important affordable waste treatment methods for small isolated industrial and community units in African country (Mahajan et al., 2009). The existence of bacterial species is more (Kogo et al., 2017), beside to the diversity of fungi-classification (Masoud et al., 2016), and algal-classification (Shreadah et al., 2015; Henry et al., 1987), in addition to protozoa-classification (Abd-Elhamied, 2020; Tembhre & Kumar, 1997), and virus-classification (Shaltout et al., 2019). The microbiological solids produced by the algae on the pond impact total suspended solids (TSS) and biochemical oxygen demand (BOD) in effluent (Tuleibah et al., 2015). Aerators used in lagoons are somewhat more costly than typical WSPs and have less efficiency at eliminating pathogens (Mara, 2001; Pearson, 1996). Temperature, dissolved oxygen (DO), and pH have a main role in oxidation ponds performance (Ali et al., 2019). WSPs (Waste Stabilization Ponds) are most infection points, including certain bacterial species, fungi, and viruses. These organisms can be killed by maturation pond. Maturation occurs after the facultative ponds, which might be a secondary or primary pond (Zimmo et al., 2003). The temperature seemed to be the most crucial physiological item influencing the performance of the ponds. Since it influences the metabolic process of the microorganisms within the system, the metabolic process is depending on inorganic nutrients stability (Alia et al., 2018).
Temperature is a physical component that influences the efficacy of the ponds as well as the metabolic rate of microorganisms in the system and thus the rate of breakdown of subsequent of inorganic nutrients and organic matter (El-Deen Ali Mohamed et al., 2004). The elimination performance of total nitrogen as ammonia, particularly in the charge of nitrification process, improved over the summer season (Masoud et al., 2004). Actually, the heavy elements cannot be digested with the assistance of the bacteria only in that frame, bacterial-algal system may block as an organic phase converted to form and bio-accumulative processes (Farr, 2004). Blood and cardiovascular (gut, liver, kidney, skin) detoxification pathways, energy-producing centers, endocrine (hormonal) controllers, enzymatic active sites, immunological, gastrointestinal, nervous (central and peripheral), urinary, and reproductive systems are among the systems in which toxic metals can cause dysfunction and impairment (Masouda et al., 2013), heavy metals components which are contributing with atmospheric deposition (Oyinloye et al., 2021), industrial effluents and spoil pile have a major role in water pollution (Hosetti & Frost, 1998).
Heavy metals such as Cu, Fe, Cd, and Zn are contaminants in aquatic environments that are widely distributed. Their reassessment stems are mostly from solid and mineral Metals such as Pb, Cd, Cr, As, Mn, Al, Fe, and Co. They have been discovered in effluent and influent samples, as well as the receiving movement. The strict laws had set, because the lives of aquatic biota and fish within the water drains are at risk, and agricultural dwellers who rely on the treated water from the water drains channels. Numerous of rural homes that deal with untreated downstream are at high-risk of poor health due to metals pollution (Oyinloye et al., 2021). Ponds can be defined as self-sufficient treatment units, because the efficacy of treatment is dependent on the preservation of the general microbial groups of fungi, viruses, protozoa, and bacteria, as well as the organics balance probability of dissolved oxygen, algae, light, and nutrient presence (Hosetti & Frost, 1998; Hosetti & Frost, 1995). Ponds can be used for “polishing” or to offer traditional treatment as well (Veeresh et al., 2010). Because of the photosynthetic activity of algae, the pond optimum depth which is detected in several plants is 44 cm deep (Epa, 2011). Because the pond is acting as shallow, photosynthetic activity supplies O2 during the day, while wind provides aeration at night (Janna, 2016). Aerobic ponds have high biochemical oxygen requirements to achieve high potential of waste removal and are in appropriate regions with low land prices to decrease the cost. These ponds have a residency time of 48 to 120 days, a BOD loading rate of 113 to 224.5 kg/1000m3 per day, and a BOD removal efficiency of 95 percent (Spellman & Drinan, 2014).
2. Experimental
2.1. Study Area
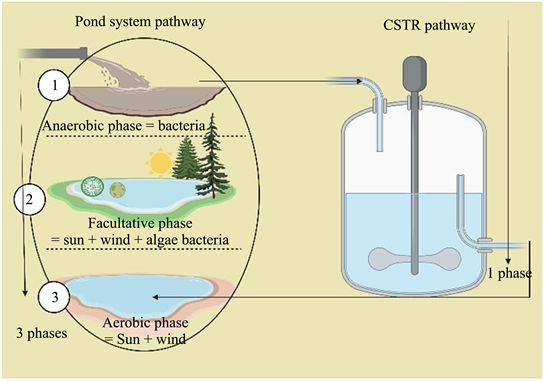
The workplace is Maturation Oxidation Ponds System at Rashid WWTP: Rashid is a city belonging to governorate of EL-Bohira, Egypt country. The maturation-oxidation pond system consists of one or two ponds, depending on the biological loads and flow; each pond is connected after the secondary clarifier; each pond has an area of 8 acres (1 acre = 4200 m2). With a capacity that varies depending on the depth of the pond (21 - 103) cm, the L-shaped pond has the proportions that had given in the image below. The pond is completely filled with secondary clarifier effluent and will retain for a period of wind responses and algal action (Figure 1 and Figure 2 and Table 1).
2.2. Sampling Program
The performance of the two treatment schemes (oxidation pond and Rashid waste water treatment plant) was monitored through the use of intensive sampling and program of testing and analysis, samples of effluent and influent from each treatment phase. All parameters included in this work were tested using the 2005 standard procedure for water and waste water assessment (Epa, 2011).
![]()
Figure 1. Diagram of Rashid WWTP maturation oxidation ponds treatment.
![]()
Figure 2. Rashid WWTP maturation oxidation ponds system place photos.
![]()
Table 1. Characteristics of rashid WWTP maturation oxidation ponds system.
2.3. Design of Oxidation Ponds as Chemical Reactor
Marais and Shaw created a Pond equation that included first-order kinetics reaction with completely mixed circumstances (Spellman & Drinan, 2014). Designers may also utilize this equation for aerobic pond design: Cn/Co = 1/(1 + kctn)n (Epa, 2011; Thirumurthi, 1974). Where Cn is the effluent BOD5 concentration in mille grams per liter; Co is the incoming BOD5 concentration in mille grams per liter; kc is the first-order reaction rate of the complete mixture flow, tn is the hydraulic residence time in each cell, in days; and n is the number of pond cells of the same size. The application of Thirumurthi contended that Marais and Shaw’s assumption of fully mixed circumstances was not optimal for aerated pond design, but chemical reaction proposals were perfect (Zimmo et al., 2003; Thirumurthi, 1974).
where:
Ce: the biological oxygen demand (BOD∙mg−1) of the effluent. Ci is the influent BOD mg∙L−1; K is the first order BOD removal coefficient, day−1; t is the mean retention time (Detention time), days;
; d = Dt/L2; d is the dimensionless dispersion number. D: axial dispersion coefficient (ft2/h). Corrections to the BOD first order (K) elimination coefficient for organic load, temperature, hazardous contaminants, and insolation. The equation K = K8CTeCoCT0x describes the components K (Farr, 2004; Oyinloye et al., 2021), where K is the first order BOD removal coefficient, Tag1; K8 is the BOD5 removal coefficient, day−1; CTe is the temperature correction factor; Co is the organic load correction factor and CT0x is the hazardous chemical correction factor; as shown in Figure 3 a simple pattern of a chemical reactor.
![]()
![]()
Figure 3. CSTR mixing patterns and CSTR reactor sketch.
2.4. Operational Conditions
Appropriate process controls must be in place to achieve high efficiency of the pond. The volume of influent should be checked on a regular basis to give the information required for proper treatment of the effluent stream and facility operation. It is also vital to monitor the operations conditions in order to control wastewater quality concerns inside the system. The inflow, pond processes (internal), and system outflow must all be checked on a regular basis. The following is an overview of some of the most critical aspects of stabilization pond operation: Light intensity, wind, temperature, pond shape, and oxygen content are the operational conditions for pond oxidation.
The operator uses the Measurements analyses, combined with indications, to determine if the pond fulfils the discharge permits such as: Temperature, pH, Organic loads (BOD5, NO2, NO3), Algae, Light Energy, Bacterial Nutritional Needs, Algal Nutrients, Physical Environment, Light Penetration, Wind, Pond Geometry, and Nutrients (ammonia and phosphorus). The ideal operating depth (H) achieved during the summer session and the presence of algae is between 0.35 and 40 meter, according to BOD5 percent beside the study schemes categorized at many operational depths. To determine the optimal operational depth based on BOD kinetics response and demonstrate pond behavior as CSTR (continuous stirring tank reactor) by comparing the pond’s BOD reactions’ K (rate constant) and the CSTR operational design equation. Marais and Shaw created a pond equation (Oyinloye et al., 2021). That incorporates both first-order kinetics and thoroughly mixed circumstances. Stabilization pond is a chemical reactor where a chemical reaction takes place in a vessel according to Thirumurthi’s instructions condition of the pond (Oyinloye et al., 2021; Hosetti & Frost, 1998). CSTR (continuous stirring tank reactor) is a type of reactor used in industrial processing; the stirred tank generally operates constantly; it is also known as a back mixing reactor and is mostly used for liquid phase processes. It is typically operated at steady state conditions and is considered to be properly mixed; hence, temperature, concentration, or reaction rate within the CSTR have no time or location dependency. That means the reactants at every location within the reactor and every variable is the same. The CSTRs are considered to be properly mixed and function in a steady state (accumulation = 0). This makes temperature, concentration, and reaction rate is independent of the reactor location. There is no buildup since CSTRs work in a steady state, and rA is position independent (Dos et al., 2017).
So, using the general mole balance equation:
(1)
We obtain CSTR equation
(2)
where V is the system volume, FA0: is the flow rate input molar (moles/time) FA: is the molar flow rate output (moles/time), rA: is the formation of species rate A per unit volume has units of moles per unit volume per unit time (i.e. concentration per time).
If ν is the flow rate volumetric (volume/time) and CA is the concentration (moles/volume) of species A,
Then
such that
(2')
If ν0 = ν
So, at 1st order reaction:
So
(2'')
So
(2''')
2.5. Determination of the K (Rate Constant) of the Pond’s BOD Kinetics Reaction
It is assumed that the rate at which the O2 is consumed has directly variation to the concentration of the reacted organic phase remaining. The kinetics of BOD reaction can be written according to first order reaction kinetics as (Thirumurthi, 1974):
where, Lt = amount of first order BOD remaining in wastewater at time t, K = rate constant of BOD reaction, time−1.
By integration:
i.e.,
(3)
or
(3)*
So,
(4)
(5)
where Lo or BODu at time t = 0, i.e., ultimate first stage BOD initially present in the sample.
The amount of BOD that has been exerted (amount of oxygen consumed) at any time t is given by: BOD exerted at t.
(6)
and the five day BOD is equal to:
(7)
Calculation the rate constant k values Using the Volumetric flow rate equation and K (rate constant) of the pond’s BOD kinetics reaction equations.
(8)
(8')
where: v = volume t = time
From Equation (5) and (8').
So, from the results which are mentioned in Table 2 that illustrate the relation between the pond Depth (H) and BOD, pond volume V, the volumetric flow ν, Rate of the BOD reaction and Rate constant of the BOD reaction K values. The rate constant, k, gives a direct measure of the relative reaction rate. So, the largest value for the rate constant equal to 4.64 * 10−6 sec−1, means a large value for the rate equal to −0.12 * 10−3 gm/m3∙sec, and that the reaction is rapidly proceeded which corresponding to the pond Depth (H) that equals to 0.3 meter. By the calculation of the rate constant k values of the CSTR according the equation number (2'''),
From the result which are mentioned in Table 2 and Figure 4: that illustrate the relation between the pond Depth (H) and BOD, V, ν, R and K values according to CSTR equation. The constant rate k is obtained by direct measurements of the rate reaction. So, the largest value for the rate constant equal to 5.24 * 10−6 sec−1, that means a large value for the rate equal to −0.13 * 10−3 gm/m3∙sec, and that explain how the reaction is rapidly proceeded which is corresponding
![]()
Table 2. Relation between the pond depth (H) and BOD5, V, ν, R and K values.
to the pond Depth (H) that equals to 0.3 meter. By comparing between the rate R and rate constant K of the pond’s BOD reactions and CSTR operational design equation:
Rate constant K Congruence percent = (100 − (|K (rate constant) CSTR − K (rate constant) BOD|/K (rate constant) CSTR)) * 100 = 88.58%
Rate Congruence percent = (100 − (|CSTR (rate R) − BOD (rate R)|/CSTR (rate R))) * 100 = 88.58%
Due to the results obtained from Table 2 which is show the optimum operational depth according to BOD kinetics reaction. By comparing between K (rate constant) of the pond’s BOD reactions and CSTR operational design equation, the Congruence percent of (the rate R and rate constant K) of the pond’s BOD reactions and CSTR operational design equation proves that the oxidation pond has a behavior as CSTR (continuous stirring tank reactor) with 88.58% Congruence percent between them. By studying the relation between BOD Reactions through the oxidation pond as a time function according to equation number (4) results which mentioned on Table 3 had obtained.
By studying the relation function of ln(concentration) as a function of the time, the first order reaction has obtained.
Demonstrating the linearity function and proving that the oxidation pond system follows the first order kinetics reactions according to equation 4 that indicates the BOD values and ln(BOD) values, as shown in Table 3 and Figure 5.
![]()
Figure 4. The data of High (H (m), ν m3/sec, BOD in and BOD out (gm/m3).
![]()
Table 3. BOD Reaction through the oxidation pond versus time according to equation number (4).
![]()
Figure 5. The relation between the time (t) hr., BOD gm/m3 and ln(BOD).
2.6. Plant Operation Included Oxidation Pond System
The influent sewage was measured at 17,000 m3/day on average. During this time, the treatment is divided into two stages. The first phase is the extended aeration treatment system, which is followed by the second step, which consists of one oxidation pond that is acting as an aerated and polishing lagoon. The DO was analyzed, and the result was 7.69 mg∙L−1 within the outlet. Eventually, the algae settle and die in the bottom of the oxidation pond; approximately 21% of the algal molecular mass is nitrogen, and no biodegradable components remain trapped within the pond settling. That biodegradable portion associated with the ultimately returns to the pond’s liquid and is recycled into the cells algae to restart the process. When the pH is too high, some of the ammonia will leave the pond via volatilization. The percentage of ammonia removed was 36.11 percent. The physiochemical parameters revealed that the influencing variables on the growth of nitrification bacteria include substrate content, DO, pH, and temperature. The elimination of nitrates, ammonia, and NO2 was 6.74 percent. Table 4 and Figure 6 show the Summery of the Physo-chemical Parameters Performance data of Nitrogen Compounds during Biochemical Nitrogen Pathway Conversion and the Summery of the Physo-chemical Parameters Performance data of Nitrogen Compounds Concentration Variation.
2.7. Heavy Metals Removal
Table 5 illustrates the impact of the combined final effluent of activated sludge system (Rashid treatment plant) and oxidation pond system on treating heavy metal-contaminated wastewater. It is critical to note that the pH of the most recent treated water, 7.57, was higher than the influent of pH at the pond, 7.42. As a result, minor enhancements such as the metal setting may also occur. It is generally understood that the solubility of the metals is governed by the value of pH. Heavy metals are often connected with solids (suspended) similarly to the co-operation manner of bacteria. The proposed removal of heavy metals, namely Cd, Pb, Zn, Cu, Mn, and Fe, was 37.82, 28.41, 39.49, 36.67, 60, and 55.71 percent.
![]()
Figure 6. The relation between the variation by area d/dt (Ammonia), d/dt (NO3) and d/dt (NO2) in raw sewage, aeration tanks, Secondary sedimentation clarifiers and Oxidation ponds effluents.
![]()
Table 4. Summary of the Physo-chemical Parameters performance data of the Nitrogen compounds concentration variation by the time during Biochemical Nitrogen pathway conversion.
![]()
Table 5. Performance results of the combined final effluent of activated sludge (Rashid) and Oxidation pond system treating domestic waste water of heavy metal Concentrations of different elements samples of treated water (mg/l) and tissues of algal (mg Kg/l).
The coefficients of bioaccumulation in active algae over the treated wastewater were determined to detect the components toxicity. The efficiency of (Chlorophyta) Green algae and (cyanophyta) blue-green Algae had obtained by divided the metals concentrations which algae have in its tissue above metal concentration in pond water. The high bioaccumulation abilities of (Chlorophyta) Green and (cyanophyta) blue-green Algae for metals selecting are illustrated by the result acquired in Table 5 and Table 6 and Figure 7.
Sketching the biochemical reaction of the maturation oxidation ponds (MOPs) is clearing that algae and Bacteria are corporately working to fulfil each other requirements. As the oxidation and reduction reactions are co-occurred, oxidation ponds are referred also as redox pond. It is worthy to clarify that this
![]()
Figure 7. Sketching the biochemical reactions through the pond.
![]()
Table 6. The algae examination effluent water average concentration, algae tissue average concentration removal percent and calculated bioaccumulation coefficients.
work confirms the previous data that clarifies the importance of scientific research in nature (Ahmed et al., 2022; Abdel-Raheem et al., 2021a, 2021b, 2021c, 2021d; Tolba et al., 2021a, 2021b; Abdel-Raheem et al., 2020; Abdel-Raheem et al., 2022a, 2022b; Bakhite et al., 2017; Kamal El-Dean et al., 2019a, 2019b; Tolba et al., 2022a, 2022b; Abdelhafeez et al., 2022; Abdelhamid et al., 2021; Elhady et al., 2022; Kaid et al., 2022; Mohamed et al., 2022; Abd-Ella et al., 2022; Gad et al., 2021; Al-Taifi et al., 2016; Bakhite et al., 2014; Fouad et al., 2023).
3. Conclusion
The oxidation treatment of ponds is based on the activity of bacteria, which convert hydrocarbons to CO2, water, Phosphate, and Ammonia in the presence of Oxygen beside to the algae’s rule of treatment. The Oxygen gas which is produced by algae during the photosynthesis process is consumed by bacteria and increases the amount of dissolved oxygen in addition to lowering organic chemicals. Performance of elimination the BOD is dependent on several parameters and has a limit of roughly 80% - 82% (Epa, 2011; Janna, 2016). The oxidation pond also depends on biological aspects rather than just the oxidation pond design itself (Dos et al., 2017).
Acknowledgements
Our deepest thanks to staff of Rashid wastewater treatment plant in Behera water and waste water company.
Funding
No financial support was given to the authors for any matter related to this article.
Appendix
Abbreviations
APHA: American Public Health Association
ASP: Activated Sludge Process
BOD5: Biological oxygen Demand
COD: Chemical Oxygen Demand
CSTR: Continuous stirring tank reactor
DO: Dissolved Oxygen
MOPs: Maturation Oxidation Ponds
TSS: Total Suspended Solid