Synthesis of Substituted Benzothiazepine Compounds with Medicinal Potential ()
1. Introduction
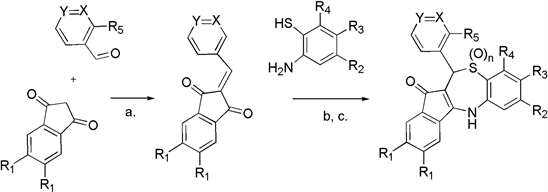
As part of an antimalarial drug development project, we wished to synthesize a series of substituted benzothiazepine compounds 1 - 14 (Scheme 1, Table 1). We expected that these would be accessible via Knoevenagel condensation of 1,3-indanediones with benzaldehydes (Scheme 1, Step a.), followed by sequential thio-Michael addition and intramolecular imine formation upon reaction with 2-aminobenzenethiols (Scheme 1, Step b.) [1]. During the course of our investigations, five articles [2] [3] [4] [5] [6] and three patents [7] [8] [9] were published that build upon the original report of this compound series by Krysin [1]. The majority of analogs described include structural variation of the benzaldehyde-derived aromatic ring, in part due to the ready commercial availability of structurally varied starting materials [2] - [9]. Preparation of analogs resulting from structural variation of the indanedione-derived aromatic ring is far fewer [4], likely because the requisite substituted indanedione starting materials are not commercially available but rather must be prepared according to literature precedent [10] [11] [12]. Just three analogs are described displaying structural variation of the 2-aminothiol-derived aromatic ring [2] [5] [8]. Since our disclosure will focus on variations of this latter group of analogs (compounds 10 - 14), our experience preparing analogs derived from pyridine carboxaldehydes (compounds 8 - 9), and our unsuccessful efforts to prepare a benzodiazepine analog of this compound series; it complements, rather than duplicates, the prior disclosures [2] - [9]. This series of substituted benzothiazepine compounds have potential for medicinal development, not only as antimalarials [2] [6]; but also for the treatment of bacterial (including methicillin resistant Staphylococcus aureus) infection [5] [8], toxoplasmosis and amebiasis [3], and polynucleotide repeat disorder [9].
2. Materials and Methods
Starting materials and reagents (obtained from Fisher Scientific, Pittsburg, PA and Aldrich Chemical Company, Milwaukee, WI, unless otherwise noted) were used as supplied. All spectroscopic and analytical data recorded for compounds were consistent with the structure proposed. Nuclear Magnetic Resonance (NMR) samples were prepared in either deuteriochloroform or deuteriodimethyl sulfoxide and recorded on a Jeol 400 MHz NMR. IR spectra were recorded on a Thermo Nicolet iS5 spectrometer with attached attenuated total reflectance (ATR). High Resolution Mass Spectrometry (HRMS) was performed by the University of Iowa High Resolution Mass Spectrometry Facility, Iowa City, IA.
1 - 14
Scheme 1. Overall route to targeted benzothiazepines 1 - 14: Step a. Piperidine, ethanol, reflux, 3 hr (Knoevenagel condensation); Step b. Benzene:acetic acid or isopropyl alcohol:acetic acid, 3 - 5 days, room temperature (sequential thio-Michael/imine formation); Step c. 30% H2O2 (aq), acetic acid, 85˚C, 3 hr for compound 2; mCPBA, CH2Cl2, room temparature, 4 hr for compound 3 (thio-oxidation).
![]()
Table 1. Substituent variations of benzothiazepines 1 - 14. (Bolded text highlights variation from parent compound 1).
Compounds not previously reported were purified until homogeneous by thin layer chromatography and having purity > 95%, evidenced by 1H-NMR spectra and integration, and 13C-NMR spectra and HRMS are provided.
The following intermediate compounds were prepared according to literature precedent: 2-benzylidine-1,3-indanedione, 2-(2-methoxybenzylidine)-1,3-inda-nedione and 2-(3-trifluoromethylbenzylidine)-1,3-indanedione [1]; 5,6-dichloro-1,3-indanedione [10] [11]; 5,6-dimethoxy-1,3-indanedione [12]; 2-(3-pyridyl-methylene)-1,3-indandione and 2-(4-pyridylmethylene)-1,3-indandione [13]; 2-amino-4-methoxybenzenethiol [14]; 2-amino-5-methoxybenzenethiol [15]. 2-Amino-6-chlorobenzenethiol and 2-amino-4-fluorobenzenethiol were purchased from Aurora Fine Chemicals LLC and used as supplied.
5,11-Dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 1
2-Benzylidine-1,3-indanedione (234 mg, 1 mmol, 1 eq) and 2-aminothiophenol (0.160 mL, 1.5 mmol, 1.5 eq) were combined in 3 mL benzene:acetic acid (3:2). After stirring at room temperature for 3 days the solvents were removed by rotary evaporation and the resulting orange solid was purified first by recrystallization from benzene, and then by silica column chromatography eluting with 2% - 5% ethyl acetate in hexanes, giving 5,11-dihydro-11-phenyl-12H-benzo [b]indeno[1,2-e][1,4]thiazepin-12-one, 1 (351 mg, 51%) as an orange solid. 1H-NMR (CDCl3): δ 7.51 (1H, d), 7.47 - 7.28 (4H, m), 7.22 (1H, m), 7.11 - 6.97 (7H, m), 6.87 (1H, m), 5.55 (1H, s) ppm; 13C NMR(CDCl3): δ 190.5, 155.3, 142.8, 141.8, 139.2, 137.0, 133.0, 131.3, 130.1, 129.0, 127.7 (2C), 127.4 (2C), 126.5, 124.8, 124.7, 121.7, 121.6, 115.7, 111.0, 46.2 ppm; IR (ATR-IR) ν 3289, 1665, 1587, 1569, 1536, 1475, 1375, 1116, 898, 754, 721, 703, 691 and 675 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C22H16NOS 342.0953; Found 342.0954.
5,11-Dihydro-10,10-dioxide-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 2
5,11-dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 1 (325 mg, 0.95 mmol, 1 eq) and 30% hydrogen peroxide solution (10 mL, 130 mmol, 135 eq) were combined in 50 mL acetic acid [16]. After stirring at 85˚C for 2 hr, the resulting mixture was poured into water, and the resulting precipitate was then recovered by vacuum filtration. Purification was by recrystallization from methanol, giving 5,11-dihydro-10,10-dioxide-11-phenyl-12H-benzo [b]indeno[1,2-e][1,4]thiazepin-12-one, 2 (151 mg, 43%) as a yellow solid. 1H-NMR (dmso-d6): δ 8.15 (1H, d), 7.80 (1H, d), 7.67 (1H, m), 7.60 (1H, m), 7.48 - 7.38 (4H, m), 7.16 - 7.04 (6H, m), 5.67 (1H, s) ppm; 13C NMR (dmso-d6): δ 191.2, 156.2, 139.5, 137.0, 135.3, 134.5, 132.9, 132.6, 131.1, 129.7 (2C), 129.5, 129.1, 128.7 (2C), 127.6, 124.7, 124.0, 121.5, 120.6, 104.5, 66.9 ppm; IR (ATR-IR) ν 3343, 1687, 1623, 1595, 1583, 1538, 1477, 1369, 1286, 1259, 1117, 1095, 1065, 901, 802, 760, 716, 696, 608 and 557 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C22H16NO3S 374.0851; Found 374.0850.
5,11-Dihydro-10-oxide-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 3
5,11-dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 1 (328 mg, 1.0 mmol, 1 eq) was dissolved in 40 mL dichloromethane and then 70% mCPBA (1.23 g, 5.0 mmol, 5 eq) was added [16]. After stirring at room temperature for 4 hr, the solvents were removed by rotary evaporation. The resulting yellow solid was purified by silica column chromatography eluting with 30% - 40% ethyl acetate in hexanes, giving 5,11-dihydro-10,10-dioxide-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 3 (148 mg, 43%) as a yellow solid, along with unreacted 1. 1H-NMR (CDCl3): δ 7.89 (1H, dd), 7.41 (2H, m), 7.42 - 6.93 (6H, m), 6.93 - 6.72 (3H, m), 6.74 (1H, d), 6.51 (1H, m), 6.20 (1H, s) ppm; 13C NMR (CDCl3): δ 193.4, 154.9, 139.0, 138.2, 135.4, 132.9, 131.8, 131.7, 131.6, 129.7, 129.5, 128.5 (2C), 127.9, 127.8 (2C), 124.2, 122.7, 120.9, 118.8, 98.2, 63.0 ppm; IR (ATR-IR) ν 1689, 1605, 1575, 1548, 1477, 1379, 1301, 1261, 1094, 1058, 1023, 897, 848, 759, 748, 718, 693 and 651 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C22H16NO2S 358.0902; Found 358.0898.
2,3-Dichloro-5,11-dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 4
2-Benzylidine-5,6-dichloro-1,3-indanedione (102 mg, 0.34 mmol, 1 eq) and 2-aminothiophenol (0.055 mL, 0.51 mmol, 1.5 eq) were reacted in 4.5 mL benzene:acetic acid (3:2), at room temperature for 3 days. Purification was by silica column chromatography eluting with 10% - 20% ethyl acetate in hexanes, giving 2,3-dichloro-5,11-dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 4 (49 mg, 35%) as an orange solid. 1H-NMR (CDCl3): δ 7.55 (1H, s), 7.41 (1H, s), 7.34 - 7.22 (3H, m), 7.17 (1H, s), 7.12 - 6.98 (5H, m), 6.90 (1H, m), 5.51 (1H, s) ppm; 13C NMR (dmso-d6): δ 187.0, 156.0, 143.4. 142.2, 139.8, 136.5, 134.2, 133.6, 133.0, 129.8, 128.5 (2C), 127.6 (2C), 126.1, 125.5, 124.8, 124.4, 122.5, 121.9, 111.0, 46.2 ppm; IR (ATR-IR) ν 3275, 3188, 3124, 1660, 1589, 1560, 1535, 1474, 1378, 1204, 1162, 900, 793, 765, 752, 721, 693, 677, 623 and 611 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C22H14Cl3NOS 410.0173; Found 410.0178.
5,11-Dihydro-2,3-dimethoxy-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 5
2-Benzylidine-5,6-dimethoxy-1,3-indanedione (148 mg, 0.50 mmol, 1 eq) and 2-aminothiophenol (0.081 mL, 0.75 mmol, 1.5 eq) were reacted in 6 mL benzene:acetic acid (3:1), at room temperature 3 days. Purification was by silica column chromatography eluting with 25% - 33% ethyl acetate in hexanes, giving 5,11-dihydro-2,3-dimethoxy-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 5 (64 mg, 32%) as a red solid. 1H-NMR (CDCl3): δ 7.65(1H, s), 7.29 (1H, m), 7.22 (1H, m), 7.18 - 7.11 (2H, m), 7.70 - 6.94 (6H, m), 6.82 (1H, m), 5.55 (1H, s), 3.86 (3H,s), 3.84 (3H, s) ppm; 13C NMR (CDCl3): δ 190.8, 155.5, 151.1, 150.2, 142.9, 142.2, 137.2, 129.0, 128.8, 127.8 (2C), 127.5 (2C), 126.7, 126.0, 124.9, 121.7, 121.6, 109.1, 106.5, 102.1, 56.8, 56.5, 46.2 ppm; IR (ATR-IR) ν 2923, 1658, 1573, 1542, 1498, 1476, 1452, 1428, 1412, 1377, 1293, 1214, 1134, 1071, 1006, 874, 809, 834, 697 and 623 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C24H20NO3S 402.1164; Found 402.1167.
5,11-Dihydro-11-(2-methoxyphenyl)-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 6
2-(2-Methoxybenzylidine)-1,3-indanedione (241 mg, 1 mmol, 1 eq) and 2-aminothiophenol (0.160 mL, 1.5 mmol, 1.5 eq) were reacted in 10 mL benzene:acetic acid (3:2), at room temperature for 3 days. Purification was by silica column chromatography eluting with 2% - 5% ethyl acetate in hexanes, giving 5,11-dihydro-11-(2-methoxyphenyl)-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 6 (150 mg, 40%) as an orange solid. 1H-NMR (CDCl3): δ 7.51 (1H, d), 7.47 - 7.28 (4H, m), 7.22 (1H, m), 7.11 - 6.97 (7H, m), 6.87 (1H, m), 5.55 (1H, s) ppm; 13C NMR (CDCl3): δ 190.4, 156.4, 155.6, 143.9, 139.5, 137.2, 133.3, 131.3, 130.5, 130.1, 129.0, 128.0, 126.8, 125.5, 124.8, 121.8, 121.4, 119.3, 115.5, 111.5, 110.6, 55.9, 40.5 ppm; IR (ATR-IR) ν 3256, 1654, 1577, 1563, 1535, 1476, 1429, 1378, 1244, 1206, 1161, 1121, 1101, 1027, 900, 849, 759, 747, 707, 615 and 555 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C23H17NO2S 372.1058; Found 372.1057.
5,11-Dihydro-11-(3-trifluoromethylphenyl)-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 7
2-(3-Trifluoromethylbenzylidine)-1,3-indanedione (1.09 g, 3.56 mmol, 1 eq) and 2-aminothiophenol (0.581 mL, 5.38 mmol, 1.5 eq) were reacted in 40 mL benzene:acetic acid (3:1), at room temperature for 3 days. Purification was by silica column chromatography eluting with 30% - 40% ethyl acetate in hexanes, giving 5,11-dihydro-11-(3-trifluoromethylphenyl)-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 7 (0.99 g, 54%) as an orange solid. 1H-NMR (CDCl3): δ 7.63 (1H, s), 7.50 (1H, d), 7.45 - 7.34 (3H, m), 7.32 - 7.12 (6H, m), 7.00 (1H, d), 6.87 (1H, m), 5.59 (1H, s) ppm; 13C NMR (CDCl3): δ 194.7, 155.7, 155.6, 143.0, 142.6, 138.9, 137.0, 132.9, 131.4, 130.8, 130.4, 130.0, 129.8, 129.4, 128.2, 125.2, 124.2, 123.9, 123.3, 121.8, 115.9, 110.0, 45.8 ppm; IR (ATR-IR) ν 1658.3, 1567.5, 1532.8, 1472.8, 1429.4, 1377.3, 1328.3, 1303.0, 1265.8, 1232.8, 1166.6, 1155.5, 1111.9, 1096.3, 1070.8, 908.8, 751.4, 729.1, 718.4, 699.9, 685.0, 656.1, 610.1, 558.8 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C23H15F3NOS 410.0826; Found 410.0823.
5,11-Dihydro-11-(3-pyridinyl)-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 8
2-(3-Pyridylmethylene)-1,3-indandione (178 mg, 0.76 mmol, 1 eq) and 2-aminothiophenol (0.123 mL, 1.14 mmol, 1.5 eq) were reacted in 7 mL benzene:acetic acid (3:2), at room temperature for 3 days. Purification was by silica column chromatography eluting with 70% ethyl acetate in hexanes. Removal of the solvent by evaporation under reduced pressure gave 5,11-dihydro-11-(3-pyridinyl)-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 8 (96 mg, 37%) as an orange solid. 1H-NMR (CDCl3): δ 8.30 (1H, d), 8.25 (1H, d), 7.56 (1H, s), 7.51 (1H, dd), 7.46 - 7.34 (4H, m), 7.20 (1H, m), 7.10 (1H, dd), 7.05 (1H, dd), 7.00 (1H, m), 6.87 (1H, m), 5.55 (1H, s) ppm; 13C NMR (CDCl3): δ 190.5, 156.0, 148.4, 147.6, 142.9, 139.0, 137.9, 137.0, 135.2, 133.0, 131.5, 130.5, 129.5, 125.2, 124.0, 123.0, 122.2, 121.8, 116.5, 109.3, 43.9 ppm; IR (ATR-IR) ν 3277, 1655, 1587, 1570, 1532, 1475, 1378, 1116, 902, 748, 727, 707, 675, 617 and 558 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C21H15N2OS 343.0905; Found 343.0905.
5,11-Dihydro-11-(4-pyridinyl)-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 9
2-(4-Pyridylmethylene)-1,3-indandione (168 mg, 0.71 mmol, 1 eq) and 2-aminothiophenol (0.116 mL, 1.07 mmol, 1.5 eq) were reacted in 7 mL benzene:acetic acid (3:2), at room temperature for 3 days. Purification was by silica column chromatography eluting with 70% ethyl acetate in hexanes. Removal of the solvent by evaporation under reduced pressure gave 5,11-dihydro-11-(4-pyridinyl)-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 9 (104 mg, 43%) as an orange solid. 1H-NMR (CDCl3): δ 8.29 (2H, d), 7.55 - 7.25 (6H, m), 7.09 (2H, m), 6.99 (2H, m), 6.91 (1H, m), 5.47 (1H, s) ppm; 13C NMR (dmso-d6): δ 190.0, 157.4, 151.6, 149.6, 149.2, 143.8, 139.7, 136.6, 133.2, 132.3, 130.8, 129.9, 125.5, 124.1, 122.9, 121.2, 119.9, 107.8, 45.1 ppm; IR (ATR-IR) ν 2922, 1665, 1599, 1571, 1542, 1472, 1384, 1316, 1200, 1115, 1063, 1001, 905, 845, 765, 748, 719, 707, 612 and 558 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C21H15N2OS 343.0905; Found 343.0907.
7-Chloro-5,11-dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 10
2-Benzylidine-1,3-indanedione (0.252 g, 1.1 mmol, 1.2 eq) and 2-amino-4-chlorobenzenethiol (0.151 g, 0.95 mmol, 1 eq) were reacted in 10 mL of 3:2 acetic acid:isopropyl alcohol, at room temperature for 5 days. Purification was by recrystallization from benzene, giving 7-chloro-5,11-dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 10 (101 mg, 25%) as an orange solid. 1H-NMR (CDCl3): δ 7.52 (1H, d), 7.47 - 7.43 (1H, m), 7.40 - 7.35 (2H, m), 7.29 (1H, d), 7.18 (1H, s), 7.14 - 6.98 (6H, m), 6.84 (1H, dd), 5.52 (1H, s) ppm; 13C NMR (CDCl3): δ 190.6, 154.8, 143.9, 141.6, 138.0, 135.3, 134.2, 133.4, 131.4, 130.3, 128.9, 128.0 (2C), 127.4 (2C), 126.8, 124.9, 124.4, 121.6, 121.4, 115.6, 46.2 ppm; IR (ATR-IR) ν 3295.6, 1667.1, 1568.9, 1526.1, 1472.5, 1452.8, 1365.2, 1309.5, 1259.3, 1181.2, 1080.2, 900.7, 873.6, 811.3, 772.2, 761.6, 717.7, 707.2, 695.1, 674.9, 641.7, 620.9, 609.6, and 567.8 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C22H15ClNOS 376.0563; Found 376.0574.
9-Chloro-5,11-dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 11
2-Benzylidine-1,3-indanedione (0.105 g, 0.45 mmol, 1 eq) and 2-amino-6-chlorobenzenethiol (0.107 g, 0.67 mmol, 1.5 eq) were reacted in 5 mL of 3:1 benzene:acetic acid, at room temperature for 5 days. Purification was by silica column chromatography eluting with 20% - 30% ethyl acetate in hexanes, giving 9-chloro-5,11-dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12- one, 11 (101 mg, 25%) as an orange solid. 1H-NMR (CDCl3): δ 7.50 (1H, d), 7.46 - 7.31 (4H, m), 7.12 - 6.98 (8H, m), 5.64 (1H, s) ppm;13C NMR (CDCl3): δ 190.2, 155.4, 144.9, 141.0, 140.8, 139.1, 133.0, 131.5, 130.4, 129.0, 127.2 (2C), 127.6 (2C), 126.8, 125.9, 124.6, 122.0, 120.4, 115.7, 111.4, 46.8 ppm; IR (ATR-IR) ν 3276, 3102, 1660, 1583, 1567, 1523, 1447, 1411, 1365, 1187, 1123, 1102, 1078, 890, 764, 751, 716, 705, 622 and 560 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C22H15ClNOS 376.0563; Found 376.0578.
7-Fluoro-5,11-dihydro-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 12
2-Benzylidine-1,3-indanedione (0.247 g, 1.1 mmol, 1 eq) and 2-amino-4-fluorobenzenethiol (0.226 g, 1.59 mmol, 1.4 eq) were reacted in 12 mL of 3:1 benzene:acetic acid, at room temperature for 5 days. Purification was by recrystallization from benzene, giving 7-fluoro-5,11-dihydro-11-phenyl-12H-benzo [b]indeno[1,2-e][1,4]thiazepin-12-one, 12 (94 mg, 24%) as an orange solid. 1H-NMR (CDCl3): δ 7.50 (1H, d), 7.45 - 7.28 (4H, m), 7.09 - 6.97 (6H, m), 6.84 (1H, m), 6.59 (1H, m), 5.51 (1H, s) ppm; 13C NMR (CDCl3): δ 190.7, 162.9, 154.7, 144.4, 141.6, 139.2, 138.7, 132.7, 131.6, 130.4, 128.0 (2C), 127.5 (2C), 126.7, 122.0, 120.3, 115.7, 112.3, 112.0, 108.7, 46.2 ppm; IR (ATR-IR) ν 3297, 1667, 1600, 1575, 1540, 1476, 1374, 1313, 1169, 1116, 1072, 953, 866, 809, 765, 715, 694, 597 and 557 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C22H15FNOS 360.0858; Found 360.0866.
5,11-Dihydro-7-methoxy-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 13
2-Benzylidine-1,3-indanedione (330 mg, 1.4 mmol, 1 eq) and 2-amino-4-methoxybenzenethiol [14] (0.210 g, 1.4 mmol, 1 eq) were reacted in 25 mL of 3:2 isopropyl alcohol:acetic acid, at room temperature for 3 days. Purification was by silica column chromatography eluting with 10% - 30% ethyl acetate in hexanes, giving 5,11-dihydro-7-methoxy-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4] thiazepin-12-one, 13 (193 mg, 37%) as an orange solid. 1H-NMR (CDCl3): δ 7.50 (1H, d), 7.44 (1H, t), 7.36 (1H, t), 7.31 (1H, d), 7.19 (1H. s), 7.09 - 6.85 (5H, m), 6.95 (1H, d), 6.60 (1H, d), 6.44 (1H, dd), 5.47 (1H, s), 3.77 (3H. s) ppm; 13C NMR (CDCl3): δ 190.9, 160.3, 155.4, 144.0, 142.0. 139.4, 138.1, 133.0, 131.4, 130.2, 127.9 (2C), 127.6 (2C), 126.5, 121.7, 115.9, 115.8, 111.4, 110.9, 107.4, 55.5, 46.3 ppm; IR (ATR-IR) ν 1662.5, 1600.1, 1573.2, 1536.1, 1479.5, 1371.8, 1284.8, 1242.3, 1194.5, 1173.5, 1117.6, 1071.5, 1029.8, 941.9, 897.5, 862.1, 836.2, 793.0, 766.2, 718.3, 694.7, 604.4, 558.7 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C23H18NO2S 372.1058; Found 372.1070.
5,11-Dihydro-8-methoxy-11-phenyl-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one, 14
2-Benzylidine-1,3-indanedione (0.411 g, 1.8 mmol, 1 eq) and 2-amino-5-methoxybenzenethiol [15] (0.373 g, 2.6 mmol, 1.4 eq) were reacted in 25 mL of 3:2 benzene:acetic acid, at room temperature for 5 days. Purification was by recrystallization from benzene, giving 5,11-dihydro-8-methoxy-11-phenyl-12H-benzo [b]indeno[1,2-e][1,4]thiazepin-12-one, 14 (213 mg, 32%) as an orange solid. 1H-NMR (CDCl3): δ 7.74 (1H, s), 7.46 - 7.43 (1H, m), 7.40 - 7.36 (1H, m), 7.35 (1H, s), 7.33 - 7.30 (2H, m), 7.18 - 6.96 (5H, m), 6.67 (1H, dd), 6.55 (1H, d), 5.55 (1H, s), 3.60 (3H, s) ppm; 13C NMR (CDCl3): δ 190.4, 156.4, 155.9, 141.8, 139.1, 135.9, 133.5, 131.2, 130.2, 128.4, 127.9 (2C), 127.6 (2C), 126.6, 126.3, 123.1, 121.6, 120.4, 115.8, 109.9, 56.0, 46.5 ppm; IR (ATR-IR) ν 3280, 1659, 1537, 1489, 1378, 1265, 1250, 1202, 1179, 1118, 1040, 899, 804, 767, 752, 720, 692, 604 and 574 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd. for C23H18NO2S 372.1058; Found 372.1064.
3. Results and Discussion
Knoevenagel condensation of commercially available benzaldehyde with 1,3-indanedione (Scheme 1, Step a), was followed by reaction of the resulting 2-benzylidine-1,3-indanedione with 2-aminothiophenol (Scheme 1, Step b). The latter entails intermolecular thio-Michael reaction, followed by intramolecular imine formation; as described by Krysin [1]. The resulting parent benzothiazepine 1 was isolated by silica gel chromatography, as a bright orange solid, in 51% yield. Unoptimized yields of the other benzothiazepine analogs 4 - 14 described herein ranged from 24% - 54%. Since we were interested in performing biological testing of the products, in our purifications, purity was maximized at the expense of yield, so optimized yields are likely higher. We found that such benzothiazepines characteristically elute from columns in relatively broadbands, and sometimes require further purified by crystallization from hot benzene. The compounds are stable for extended periods when stored as solids at room temperature. Their stability is such that long-term storage as DMSO solutions (kept in the freezer), and short-term storage in various solvents (during purification and analysis) is possible.
Given that our interest in the benzothiazepine compound series was for medicinal application, and the potential that a sulfur atom may be oxidized in the presence of metabolizing enzymes; we were keen to explore the synthesis of both the sulfone 2 and sulfoxide 3 analogs of the parent compound 1. Following the procedures developed by Lévai for oxidation of 1,5-benzothiazepin-4(5H)-ones, oxidation of the thioether group of parent benzothiazepine 1 (n = 0) with 30% hydrogen peroxide solution afforded the novel sulfone analog 2 (n = 2), as a bright yellow solid [16]. Alternatively, oxidation with 70% mCPBA (requiring a significant excess of oxidant compared to that used by Lévai [16] ) afforded the bright yellow novel sulfoxide analog 3 (n = 1), as an inseparable mixture of diastereomers.
We did explore the possibility of replacing the sulfur atom of the parent benzothiazepine 1 with a nitrogen, to entirely avoid the potential for metabolic thio-oxidation. However, despite literature precedent [8] [17], and repeated effort, we were never able to isolate the corresponding benzodiazepine analog from the reaction of 2-benzylidine-1,3-indanedione with 1,2-diaminobenzene. Proton NMR of crude reaction mixtures appeared to contain trace quantities of the targeted benzodiazepine (evidenced by the characteristic shift of the 11-hydrogen around δ 5.6 ppm), but attempted purification invariably led to loss of this signal.
To explore substitution on the indandione-derived portion of the compound, we prepared 5-chloro-1,3-indanedione [10] and 5-methoxy-1,3-indanedione [18] according to literature precedent, and then the corresponding benzylidines using the method employed by Krysin [1]. Upon reaction with 2-aminothiophenol each benzylidene afforded an inseparable mixture of 2-substituted and 3-substituted benzothiazepine regioisomers. (Sieber reports similar mixtures, and also several analogs where separation of regioisomers was possible [5] ). To circumvent this issue, we prepared the corresponding disubstituted starting materials, 5,6-dichloro-1,3-indanedione [11] and 5,6-dimethoxy-1,3-indanedione [12]. Separate reaction of the corresponding benzylidenes with 2-aminothiophenol yielded the novel 2,3-dichloro-substituted benzothiazepine 4 and the novel 2,3-dimethoxy-substituted benzothiazepine5, respectively.
As others have reported [1] - [9], synthesis of benzothiazepines bearing structural variation of the benzaldehyde-derived aromatic ring is readily achieved, and is demonstrated by our preparation of compounds 6 [2] and 7 [5]. Wanting to enhance the overall polarity of the compound series, we sought to incorporate 11-pyridyl-substitution. We prepared 2-(2-pyridylmethylene)-1,3-indandione, 2-(3-pyridylmethylene)-1,3-indandione, and 2-(4-pyridylmethylene)-1,3-indandione according to literature precedent [13]; and attempted reaction of each with 2-aminothiophenol. Benzothiazepine synthesis was successful in the cases of the 11-(3-pyridyl)-analog 8 [5], and the novel 11-(4-pyridyl)-analog 9; but despite repeated attempts, we were unable to prepare the corresponding 2-pyridyl-analog, for reasons we were unable to ascertain.
Just three analogs have been previously described where structural variation is in the aminothiol-derived aromatic ring [2] [5] [8], and these are limited to 8-methoxy or 8-methyl substitution. We initially targeted two novel methoxy-substituted analogs 13 and 14. Their synthesis required preparation of 4-methoxy- and 5-methoxy-substituted 2-aminobenzenethiol, from 2-amino-5-methoxy-1,3-benzothiazole [15] and 6-methoxy-2-methyl-1,3-benzothiazole [16] respectively, according to literature precedent. These substituted 2-aminobenzenethiols were separately reacted with 2-benzylidine-1,3-indanedione, to yield novel 7-methoxy substituted benzothiazepine 13 and novel 8-methoxy substituted benzothiazepine 14. Additionally, we used commercially available 4-chloro-, 6-chloro-, and 4-fluoro-substituted 2-aminobenzenethiols in separate reactions with 2-benzylidine-1,3-indanedione, producing three novel halo-substituted analogs: the 7-chloro-substituted analog 10, the 9-chloro-substituted analog 11, and the 7-fluoro-substituted analog 12. Analogs 10 - 13 are particularly significant, since they represent the first report of compounds in this class of benzothiazepine bearing substitution at the 7- or 9-position (R2 and R4, respectively).
4. Conclusion
The 5,11-dihydro-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-one(benzothiazepine) scaffold is reported to have a range of biological activities, and several publications have described the synthesis of analogs of this compound series [2] - [9]. This article describes a more detailed investigation of structural variation than those reported to date. Structural modification at the sulfur atom in the parent benzothiazepine 1 was achieved by oxidation [16], yielding sulfone 2 and sulfoxide 3; though synthesis of the corresponding benzodiazepine was unsuccessful, despite literature report saying otherwise [17]. A pair of novel 2,3-disubstituted benzothiazepines 4 and 5 are described, along with a pair of analogs bearing substitution on the 11-phenyl-substituent, compounds 6 and 7. Of potentially greater interest (given the probably excessive lipophilicity of the parent benzothiazepine for medical application), are the more polar, 11-pyridyl-containing analogs 8 and 9. Novel compound 9 is of particular note since it represents the first report of an 11-(4-pyridyl)-substituted analog. Our finding that the corresponding 11-(2-pyridyl)-substituted analog is inaccessible by this synthetic route is also noteworthy. Lastly, a significant expansion (compared with those described in the literature) of analogs substituted on the aminothiol-derived portion of the benzothiazepine series is described. Compounds 10 - 12 are the first halo-substituted analogs described; compounds 10, 12 and 13 the first bearing substitution at the 7-position; and compound 11, the first at the 9-position. We expect that these findings will assist the further development of 5,11-dihydro-12H-benzo[b]indeno[1,2-e][1,4]thiazepin-12-ones, not only as antimalarials [2] [6]; but also for the treatment of bacterial (including methicillin resistant Staphylococcus aureus) infection [5] [8], toxoplasmosis and amebiasis [3], and polynucleotide repeat disorder [9].
Acknowledgements
We are grateful for support of this work by the Office of Naval Research, the Naval Academy Research Council of the United States Naval Academy, the Walter Reed Army Institute of Research, and the Service Academy Initiative of the Defense Threat Reduction Agency. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official, or reflecting true views of the Department of Defense, the Department of the Army or the Department of the Navy.