Values of the second thermodynamic dissociation constant pK
2 of the protonated form of monosodium 1,4-piperazinediethanesulfonate (PIPES) have been determined at twelve different temperatures in the temperature range from (278.15 to 328.15) K including the body temperature 310.15 K by measurement of the electromotive-force for cells without liquid junction of the type: Pt (s), H
2 (g), 101.325 kPa|Na-PIPES (m1) + Na
2-PIPES (m
2) + NaCl (m
3)|AgCl (s), Ag (s), where m
1, m
2 and m
3 indicate the molalities of the corresponding species at 1 atm = 101.325 kPa in SI units. The pK2 values for the dissociation of Na-PIPES are represented by the equation: pK
2 = -1303.76/T + 48.369 - 6.46889 lnT with an uncertainty of ± 0.001. The values of pK
2 for Na-PIPES were found to be 7.1399 ± 0.0004 at 298.15 K and 7.0512 ± 0.0004 at 310.15 K, respectively, and indicate that this buffer would be useful as pH standard in the range of physiological application. Standard thermodynamic quantities
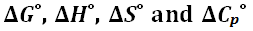
for the acidic dissociation process of Na-PIPES have been derived from the temperature coefficients of the pK
2. These values are compared with those of structurally related N-substituted PIPERAZINE and TAURINE at 298.15 K.