A Unique Conformational Behaviour of Glutamine Peptides ()
ABSTRACT
Trinucleotide repeat expansions (CAG) lead to increase in glutamine residues and hence increase in glutamine stretch. This leads to number of neurodegenerative diseases. Therefore, the conformation of poly Q of varying chain lengths has been investigated by quantum mechanical and molecular dynamics approaches. Glutamine contains amide linkage in the side chain. It is the interaction between side chain amide linkage and the peptide bond of the backbone which dictates the conformational behaviour. Some of the glutamine residues adopt phi psi values corresponding to poly-L-proline type II structure. Not more than three glutamine residues are found to have the same set of
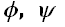
values and hence polyglutamine is adopting random coil structure. Carbonyl-carbonyl, CH-O interactions and hydrogen bond formation involving backbone and side chain amide chain linkages are found to contribute to the stability of the adopted structure. In simulation studies due to interaction of water molecules with the amide linkages the
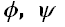
values undergo change and this leads to weakening of carbonyl-carbonyl interactions and hydrogen bonds. The conformational behaviour of polyglutamine peptides is shown to be chain length dependent and this may provide some insight regarding the aggregation behaviour of proteins containing poly Q stretch. Possibly this is the first systematic study of the conformational behaviour of polyglutamine peptides.
Share and Cite:
Baskar, M. , Shafique, M. and Nandel, F. (2014) A Unique Conformational Behaviour of Glutamine Peptides.
Journal of Biophysical Chemistry,
5, 33-43. doi:
10.4236/jbpc.2014.52005.
Cited by
No relevant information.