Synthesis of Geopolymers Using Local Resources for Construction and Water Purification ()
1. Introduction
Geopolymers have drawn attention because of their great mechanical characteristics. It has been observed that through chemical polymerization reactions, aluminosilicates such as clays could be hardened and transformed into aluminosilicate polymers, also known as geopolymers, which are potential construction materials [1] [2] . Geopolymers consist of an amorphous, three-dimensional structure resulting from the polymerization of aluminosilicate monomers in an alkaline solution [3] , aluminosilicate monomers in an alkaline solution [3] .
The exact mechanism of geopolymerization is not known precisely until now. There is a good probability for Na+ ions released from Na-alkali solution to replace hydrogen ions on the broken edges of the clay. As a result of this ion exchange, repulsion between the Na+ ions will transform the two-dimensional phyllosilicates sheets into three-dimensional framework of tectosilicates [4] . Davidovits [5] has proposed a reaction pathway for geopolymerization involving polycondensation of hypothetical monomers, i.e. orthosialate ions [4] . Davidovits [5] has proposed a reaction pathway for geopolymerization involving polycondensation of hypothetical monomers, i.e. orthosialate ions. As a result of these reactions, solid, hard, and stable materials similar to hydroxysodalite, feldspathoid, or zeolite are formed [2] . When aluminum is four coordinated coordinated to oxygen atoms, a negative charge is created and therefore the presence of cations, such as Na+, is essential to balance electric charge in the geopolymeric matrix (hydroxysodalite). Hydroxysodalite, which maybe amorphous or microcrystalline, consists of SiO4 and AlO4 tetrahedra linked alternately by sharing all the oxygen atoms [6] . Positive ions (Na+, K+, Li+, Ca2+, Ba2+, NH4+, and H3O+) need be present in the framework cavities to balance the negative charge of Al in the fourfold coordination.
Little work was found in the published literature on the adsorption potential of geopolymers. Li et al. [7] studied the adsorption of methylene blue (MB) dye onto geopolymeric adsorbent based on fly ash. The synthesized geopolymer exhibited much higher adsorption capacity towards MB than fly ash itself. Wang et al. [8] prepared an amorphous aluminosilicate geopolymer through solid-state conversion of fly ash. The synthesized geopolymer was also found to have higher adsorption capacity towards Cu2+ ion (92 mg Cu/g adsorbent) than the fly ash itself (0.1 mg Cu/g adsorbent).
In order to combat large-scale pollution in many parts of the world caused by micropollutants, low cost materials with suitable mechanical properties and high adsorption capacity are required [9] . Geopolymers produced from local resources are considered as candidate materials for this purpose. They may be also used for the construction of water storage or transport facilities (e.g. pools, dams, channels) and as liners in landfills to improve leachates quality and minimize thus the risk for groundwater and soil contamination [10] [11] .
This research focuses on preparation and evaluation of functional metakaolin-zeolitic tuff geopolymers using local resources from Jordan, in terms of microstructure, mechanical properties and potential for wastewater purification. Zeolitic tuff is a potential candidate material due to its availability and high adsorption capacity. The adsorption potential of the produced geopolymers towards Cu2+ ions is investigated.
2. Materials and Methods
Materials: The geopolymers were synthesized using low purity metakaolin, natural zeolitic tuff (JZ), and alkaline activators, namely sodium silicate (Na2SiO3) and sodium hydroxide (Merck, Germany). The zeolitic tuff was from North-East Jordan deposits (Aritayn area) located around 50 km to the east from Amman, the capital. The mineral composition of this tuff belongs mainly to phillipsite type of zeolites [1] [11] . Table 1 shows the chemical composition of kaolinite and zeolitic tuff. JZ was pulverized, sieved through 450 μm sieve, and exhibited a median grain size (d50) of 70 μm. Jordanian kaolin with a purity of 60% [1] was from El-Hiswa deposit, which is located in the southern of Jordan about 45 km to the east of Al-Quweira town [12] . The elevation of the upper surface deposit ranges between 850 and 950 m above sea level. Thirty-two individual samples were collected from different 8m of the upper layers of an outcrop, weighing in total 32 kg.

Table 1. Chemical composition of kaolinite (JK), and zeolitic tuff (JZ) [1] .
Slopes range between 1% and 5% and soils are highly calcareous, shallow on higher slopes, deeper on lower slopes and on gradients of less than 2%. Soil texture is generally silty clay soil that show poor physical structure with relatively good fertility status. Soil surface is prone to crusting, erosion and water runoff where terminal rates of infiltration rate range 3 - 14 mm/h [12] . Soil is generally susceptible to dispersion and capping which leads to very high proportions of runoff. Rainfall events of less than 5 mm can cause noticeable runoff flows. Rainfall is irregular and unpredictable occurs in the form of short, intensive storms between November and May, with a long-term average of less than 200 mm per annum. Only a small quantity of rain is retained by soil surfaces and most is lost either through direct evaporation from the surface or through runoff into gullies.
Production of geopolymers: The variables considered to investigate the alkaline activation process were: SiO2 (in sodium silicate solution)/Al2O3 (in metakaolin) molar ratio of 1, and Na2O (in sodium silicate and NaOH solutions)/Al2O3 (in metakaolin) molar ratio of 1. The H2O/Na2O molar ratio was 16. The weight ratio of zeolitic tuff to metakaolin is 0.6. The aqueous solution with Stoichiometric amount of Na2SiO3, NaOH and H2O were mechanically mixed for 1 min. The zeolitic tuff and metakaolin were dry mixed first and then the sodium hydroxide and sodium silicate solutions were added. All reagents were mixed for 15 minutes. The final pulp from each series was poured into three rectangular molds (12 cm × 2 cm × 4 cm each). The molded specimens were cured in a ventilated oven at 40˚C for 24 h. After curing, specimens were removed from the molds and cooled at room temperature. The specimens were subjected to three point bending, and compression tests. In addition, nine discs (2 cm × 2 cm × 1 cm) were prepared from each mixture, to carry out mineralogical studies using Xray diffraction (XRD), and scanning electron microscopy (SEM) as well as to assess the adsorption capacity of the produced geopolymers.
Mineralogical analyses: XRD analyses were carried out on powdered samples of geopolymers to identify the dominant crystalline and potentially newly formed phases. The XRD patterns were obtained from 4˚ to 40˚ 2θ at a scan rate of 2˚/min.
Morphology of the specimens was also studied with an Inspect F50 scanning electron microscope (Japan). The samples were pre-coated with platinum under an argon atmosphere.
Geopolymer properties: Three specimens (10 cm × 2 cm × 4 cm each) were tested for the three point bending strength under ambient conditions. The standard formula for strength in 3-pt bending of a specimen is used:

Here P is equal to the failure load, L is the support span, d is the sample height, and b is the sample width. The specimen’s dimensions are: height = 20 mm, width = 40 mm and length 100 mm, the distance between the supports is 80 mm and the speed of the machine head during testing is 0.1 mm/minute. The compression tests were performed on three specimens with dimensions of (2 cm × 2 cm × 4cm of each. All tests were carried out in two replicates. The speed of the machine head during testing is 2 mm/minute. Compressive strength is obtained by dividing the failure load by the cross section area.
In order to assess the adsorption potential of the produced geopolymers, a group of geopolymeric discs from each composition immersed, and washed once everyday with distilled water for 4 days. The aim of this procedure is to remove the residual water-soluble salts from the geopolymers. Discs were immersed in standard solution (250 mg/L) of cupric acetate (98%, Hopkin and Williams) in 0.1M NaClO4 solution (98) at pH = 3 (pH adjusted using NaOH/HClO4). Ten milliliter samples from each solution were withdrawn after 24 h of shaking, filtered through microfilters (0.45 μm Nylon), and centrifuged prior to the determination of Cu2+ concentration using atomic absorption spectrometer (Spectroscan-80DV-Germany).
3. Results and Discussion
Micro structural characteristics: Figure 1 shows the XRD patterns of kaolin, zeolitic tuff and the geopolymers produced. The results show that kaolin contains mainly kaolinite and Quartz. Zeolitic tuff contains mainlyphillipsite as well as calcite, and kaolinite.
During metakaolin-alkali geopolymerization phillipsite disappeared due to dissolution in alkaline geopolymerisation environment as shown in the XRD pattern of the geopolymer. The high XRD background seen between 20˚ to 40˚ indicates the presence of amorphous phases [13] . The microstructure of geopolymers, presented as a SEM image Figure 2(a) and Figure 2(b), shows the coexistence of geopolymer gel, amorphous phase of residual zeolitic tuff (A), and partially unreacted metakaolin sheets (B). According to EDX elemental analysis, the average Si/Al ratio in the geopolymeric gel is 2.1, while the respective ratio in the partially transformed metakaolin was lower (Si/Al ~ 1.8). The SEM image reveals that due to geopolymeric reactions the gaps between partially reacted metakaolin sheets have been filled with the formed of sodium aluminosilicate. This finding
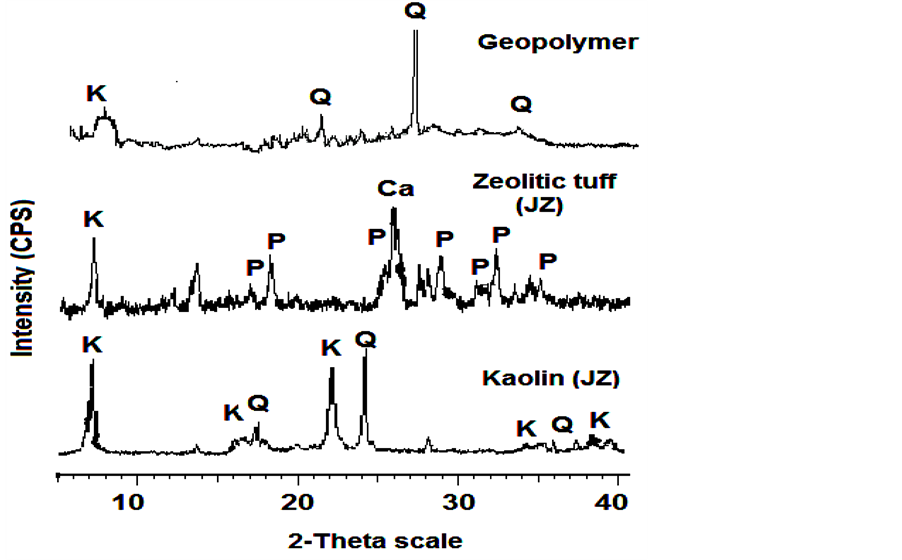
Figure 1. XRD patterns (qualitatively) of powdered kaolin (JK), zeolitic tuff (JZ), and geopolymers (K: kaolinite Al2Si2O5(OH)4, Q: quartz SiO2, Ca: calcite CaCO3, P: phillipsite K6.Al6Si10O32·12H2O).
coincides with the results published by Rahier et al. [14] , who obtained partially amorphous sodium aluminosilicates after activation of metakaolin with sodium silicate-activated.
The size of the aluminosilicate particles (~40 nm) encapsulated in a geopolymer gel determines the pore parameters observed in the microstructure of the formed products. An array of larger particles results in larger pore area and mean pore diameter. The opposite is seen for a gel network consisting of smaller particles. The formation of a uniform pore structure in aluminosilicate particles through sol-gel chemistry is also commonly observed [15] . The particle size can then be easily modified by changing the reaction temperature, curing time, and silicate ratio, as has also been observed in metakaolin-based geopolymer gels..
The precursor (zeolitic tuff) was attacked by the alkaline solution during geoplymerisation as shown in Figure 2(A), Figure 2(C) and Figure 2(D), and was partially consumed. Its residual was amorphous, as seen in Figure 2(B) Figure 2(C). Due to the alkaline attack, macro holes filled with new formed Na-alu-minosilicates products are widely scattered in the residual zeolitic tuff aggregate (B). Intensive silica zones (E) were observed in Figure 2(B). These Silica rich zones, as shown by the EDX, seem to consist of quartz as shown by the XRD patterns in Figure 1. Upon geopolymerization, crystals growth can be observed in Figure 2(B) Figure 2(C). Figure 2(B) shows the formation of new microcrystalline (E) phases formed on the surface of the amorphous residual zeolitic tuff.
Mechanical properties of geopolymers: The tensile bending strength and the compressive strength of the geoplymers are 7.8 MPa and 45 MPa, respectively as shown in Figure 3. These strength values are comparable with metakaolin-based geopolymers [4] . The participation of JZ in geopolymerization reactions, and the formation of new binding mineral matrix could have influence on improving the mechanical performance of the final geopolymeric products [10] [12] . Usually the improvement in mechanical properties correlated with the increase in the volume of geopolymeric gel at a relatively constant nominal density, results in a more homogenous microstructure. The evidence presented here shows that strength and microstructure of geopolymeric materials are altered partially by adding zeolitic tuff as reactive material. It was reported [16] that there is an increase in the compressive strength of geopolymers formed from metakaolin by addition of silica to sodium-based alkali activating solution. The potential Si source in this study is the zoelitic tuff, which dissolves in alkaline solution as shown in Figure 1. However, other factors such as the change in liquid/powder ratio and the grain packing of the geopolymer mixture could influence the mechanical properties of the geopolymers.
Adsorption efficiency of geopolymers: The adsorption efficiency of geopolymers discs was investigated in terms of adsorption of Cu2+ ions from a copper solution containing 250 mg/L Cu. Geopolymeric discs showed significant adsorption rates reaching 9.3 mg Cu/g. This value is comparable with that of a powdered zeolitic tuff used in a previous work (zeolitic tuff 8.5 mg /g, pH 4) [11] . Although the pH was adjusted at 3, in which the adsorption capacity of the zeolites is very low [17] compared with neutral conditions, the adsorption of the MZ-geopolymers is still relatively high. This is evident that these geopolymers can be used for adsorption of heavy metals ions even under extreme acidic conditions.

Figure 3. Compressive strength and tensile-bending strength of the geopolymers.
It was found that the produced geopolymer from low purity metakaolin and natural zeolitic tuff exhibits high removal efficiency of Cu(II) when compared with other minerals [11] . The formation of nano-porous mineralmatrix upon the geopolymerization increases the efficiency of the adsorption capacity. Zeolite has different sites available for adsorption of heavy metal ions and also has a cage-like structure suitable for ion exchange [18] . However, the adsorption of metal ions onto zeolite particles is a complex process because of their porous structure, inner and outer charged surfaces, mineralogical heterogeneity, crystal edges, and other imperfections on the surface [19] [20] . The incorporation of zeolite with metakaolin revealed that the produced geopolymers show a high adsorption capacity. The XRD pattern of geopolymers showed that all the phillipsite peaks of zeolitic tuff were disappeared upon geopolymerization (Figure 1). Thus, geopolymerization destructs crystals of zeolite leading to more open amorphous structure (Figure 2(a)) which leads to an increase of the number of adsorption sites. The adsorption mechanism of Cu2+ can be proposed as cation exchange of Na+ with Cu2+. This is justified as the geopolymerization process was made under highly alkaline solution of NaOH, the dispersion of aluminosilicate particles is due to Na+ exchange with the hydrogen ions of the broken edges of aluminosilicate, and the Na ions play an important role in electrical neutralization of the resultant geopolymeric matrix.
4. Conclusion
Metakaolin-Zeolitic tuff geopolymer produced from Jordanian resources has been successfully synthesized by alkali-activation. The product comprised amorphous phases and quartz as revealed by XRD analysis. This study clearly shows the influence of zeolitic tuff on the microstructure, strength and adsorption behavior of geopolymers. The results of the conducted experiment showed that this type of geopolymers exhibits high mechanical performance and high adsorption capacity toward micropollutants and cost effective. The microstructural observations using SEM reveal that the geopolymer consists of a nano-particle matrix with nanosized pore characteristics.
NOTES
*Corresponding author.