Nitrogen Fertilization Impacts on Phosphorus Cycling in Grazed Grass-Legume Pasture ()
1. Introduction
Animal manures have been used for millennia as a source of nutrients for crops of all types. With the advent of modern animal agriculture, manure-derived nutrients have become concentrated in production locales that cannot fully assimilate them. Nutrients such as P are potentially a major concern when considering quality of water runoff from beef cattle operations. When using manure from an animal feeding operation, manure is evenly spread across a pasture using a mechanical spreader or irrigation. However in grazed systems, urine and feces are not uniformly distributed throughout the pasture. A single beef-cow dung patch covers approximately 0.06 m2 [1] ; and when calculated on an annual basis, the total surface area of a pasture that receives dung may only be 27% to 40% of the total area [2] .
Cattle consuming forage-based diets require a daily minimum of 10 mg P/kg BW (body weight). The true absorption of dietary P is less than 68%, resulting in at least one-third of total ingested P being directly excreted in feces from the animal [3] . The concentration of P in forages ranges from approximately 0.3% of dry matter (DM) in early growth to 0.15% in mature growth, and may be deficient for cattle subsisting on mature, dry forages. However, certain species (e.g., tall fescue, bermudagrass, and clovers) are able to bioaccumulate P [4] [5] . Furthermore, studies have shown that these P bioaccumulators exhibit both greater biomass production and greater foliar concentrations of P when adequate N fertilization is used [6] [7] .
Only a small amount of endogenous P (approximately 0.27 g P/d) is excreted in the urine by cattle [8] [9] . Therefore, feces is a sink for both biologically unavailable and available P in the diet, and the amount excreted is largely dependent on dietary intake [10] -[12] . Reference [13] reported cattle grazing pasture excreted 4.3 - 7.3 mg P/g fecal DM and at least 13.7 kg P were excreted annually per animal. Alternatively, fecal N loss is only approximately 0.6% of DM intake by the animal, with the majority of N being excreted through the urine [8] . The resulting N:P ratio of cattle fecal material is thus much narrower than that of the forage requirement and often leads to a buildup of P in the soil [14] . It has been estimated that soil P accumulation rates due to manure application in the USA and several European countries ranges from 8 to 40 kg P ha−1∙yr−1 [15] . And while N and P in animal excreta are present in various forms, the majority of N is in excreta occuring in organic forms and P primarily in inorganic forms. Thus suggesting mineralization rates and nutrient availability in excreta depend on the nutrient and environmental conditions, such as soil moisture and temperature [15] [16] .
While many studies [17] -[20] have determined the mineralization/return rate of P from cattle manure (feces, urine, and/or bedding material) to the soil, there is little research published that deals singularly with cattle feces, which can oftentimes be N-limiting due to the small amount of endogenous N that is excreted in feces. Additionally, the return of P and N to the soil from fecal pats is affected by many environmental factors (e.g., air and soil temperature, soil moisture, etc.); thus, there is no universal standard by which to quantify mineralization, and location-specific estimations are required for individual physiographic and climatic regions [21] . The objective of the current study was to determine the effect of N fertilization of a year-round, grazed, grass/legume pasture on intake and fecal excretion of P by grazing cattle, and to characterize temporal patterns of P return to the soil ecosystem from fecal pats. We hypothesized that N fertilization would alter aboveground forage DM mass and foliar P concentration such that P intake and thus fecal excretion of P by cattle would be modified. Secondly, we hypothesized that pasture response to N fertilization would affect temporal patterns of disappearance of fecal pats such that feces from cattle grazing during the summer would disappear more rapidly and result in greater soil N and P concentrations than feces from cattle grazing during cool season.
2. Materials and Methods
2.1. Research Site
The experimental site comprised six instrumented runoff plots constructed in 2007 at the Stanley P. Wilson Beef Teaching Center of the Auburn University Department of Animal Sciences, Auburn, AL USA (32˚53'34.43"N latitude, 85˚30'3.32"W longitude, 187 m above MSL). Plots (91.4 × 30.5 m, 0.28 h each) ranged from 1 to 10% slope and consisted of a permanent common bermudagrass (Cynodon dactylon) and tall fescue (Lolium arundinaceum) sod on a Marvyn loamy sand (Fine-loamy, Kadinitic, thermic Typic Kanhapludult) and a Pacolet sandy loam (Fine, Kaolinitic, thermic Typic Kanhapludult) soil. Monthly mean and 20-yr average monthly mean temperatures at the research site between September 2010 and December 2012 are presented in Figure 1, and corresponding monthly total and 20-yr average monthly total precipitation during the same period are presented in Figure 2.
![]()
Figure 1. Monthly mean and 20-year average monthly mean temperatures between September 2010 and December 2012 at the Auburn University Stanley P. Wilson Beef Teaching Unit, Auburn, AL, USA.
![]()
Figure 2. Monthly and 20-year average monthly total precipitation between September 2010 and December 2012 at the Auburn University Stanley P. Wilson Beef Teaching Unit, Auburn, AL, USA.
2.2. Forage Establishment and Fertilization
In October 2010 and 2011, plots were no-till drilled (Great Plains model 3P606NT with small seed box) with triticale (×Triticosecale rimpaui Wittm. var. “Trical 2700”) at a seeding rate of 125.5 kg/ha and with pre-inocu- lated crimson clover (Trifolium incarnatum var. “Dixie”) at a seeding rate of 33.6 kg/ha, and randomly assigned to 1 of 3 treatments (2 replicates/treatment): 0% of N (0 N) recommendation for triticale, 50% of N (50 N) recommendation for triticale (50.4 kg N/ha) in a single application at planting, and 100% of N (100 N) recommendation for triticale (100.8 kg N/ha) in a split-application; the first at planting, and the second directly prior to turning out cattle into plots for grazing. Plots received 50.4 kg N/ha in the form of ammonium sulfate-urea at the time of planting, and 100 N plots received an additional 50.4 kg N/ha in February of each year. In May, after completion of the cool season (CS), plots were mowed to a 10-cm height using a mechanical mower to remove any standing forage. In June 2011 and 2012, plots were no-till drilled with cowpea (Vigna unguculata var. ‘Iron Clay’) at a seeding rate of 57 kg/ha. Cowpea seeds were inoculated prior to planting with N-Dure EL Type rhizobial inoculant (Intx Microbials, LLC, Kentland, IN) at a rate of 170 g inoculant per 23 kg seed. Plot assignments to fertilization treatments were the same as in the CS; for the warm season (WS), N fertilization rates were based on recommendations for bermudagrass. The 0 N treatment thus received 0 kg N, the 50 N treatment received 56.0 kg N/ha at time of planting, and the 100 N treatment received 112.0 kg N/ha in a split-application at planting and prior to turning out cattle into plots for grazing. No pastures received irrigation or pesticide application during the experimental period.
2.3. Cattle Management
For both grazing seasons in 2011, six Red Angus × Beefmaster cattle (4 steers and 2 heifers, 328 ± 60 kg BW in CS; and 6 steers, 361 ± 23 kg BW in WS) were randomly assigned to plots (1 animal/plot) and turned in to graze on February and July. Cattle were allowed to graze until forage samples from destructive harvests revealed that aboveground forage DM mass had decreased below 500 kg DM/ha (May and September, 2011). In 2012, six Angus cattle (4 steers and 2 heifers, 345 ± 60 kg BW during CS; 6 steers, 312 ± 31 kg BW during WS) were randomly assigned to plots (1 animal/plot) and turned in to graze in late-January and early August. Cattle were removed from plots when forage DM mass had decreased below 500 kg DM/ha (May and September). All animal activities were approved by the IACUC committee prior to the experiment.
2.4. Chromium Pellet Fabrication
In August 2010, chromic oxide (Cr2O3)/corn pellets were fabricated for administration to grazing cattle in order to indirectly determine their daily fecal DM output by Cr dilution technique. A mixture of Cr2O3 and ground corn was blended in a ratio of 20.4 kg corn:2.3 kg Cr2O3 and fabricated into pellets containing 6.8% Cr by weight. Pellets were formed using a laboratory-scale pellet mill (Model CL5, California Pellet Mill Co., San Francisco, CA) through a 4.76-mm dye. Pellets were dried for 24 h at room temperature and stored in an air-tight container until use.
2.5. Phosphorus Intake and Fecal Excretion
Daily forage DM intake was estimated by first determining fecal excretion of Cr from consumption of Cr2O3/ corn pellets. Cattle were individually fed 50 g ground corn twice daily for 10 d to familiarize them with hand- feeding by personnel. Next, cattle were individually fed 10 g/d of Cr2O3/corn pellets for 7 d at 0800 h. On day 7, each animal was moved at 0800 h to a holding facility at the Stanley P. Wilson Beef Teaching Unit where feces were collected from a concrete floor immediately following excretion and placed into a bucket. All feces from 0800 to 1500 h from each animal were mixed, and a 500-g subsample (wet wt) was retained for Cr analysis. This experiment was conducted twice, midway and late in the grazing season, for each of CS and WS seasons in 2011 and 2012. Prior to analyses, fecal samples were dried at 60˚C for 72 h and ground with a Wiley Mill (Thomas Scientific, Philadelphia, PA) to pass a 1-mm screen.
Three forage samples were obtained by destructive harvest in each plot within 7 d subsequent to fecal collections. Forage samples were clipped at 5 cm above ground surface from within a 0.25-m2 quadrat, composited by paddock, dried at 60˚C for 72 h, and ground with a Wiley Mill (Thomas Scientific, Philadelphia, PA) to pass a 1-mm screen. Forage in vitro dry matter digestibility (IVDMD) was determined according to the [22] modification of the procedure developed by [23] using the Daisy II incubator system (Ankom Technology, Macedon, NY). Ruminal fluid was collected from a fistulated, dry Holstein cow at the Auburn University College of Veterinary Medicine. The cow was fed a corn silage-based diet containing cottonseed meal and Megalac supplement (Arm and Hammer Animal Nutrition, Ewing, NJ), and had free access to bermudagrass pasture and limited alfalfa (Medicago sativa) hay. Fluid was stored in pre-warmed thermos containers to maintain viability of the rumen microbial population and transported immediately to the Auburn University Ruminant Nutrition Laboratory where it was processed for the batch-culture IVDMD procedure.
Forage and fecal samples were analyzed for P and Cr using dry-ashing followed by ICAP spectroscopy [24] . Total fecal Cr was used to determine total fecal output using the equation 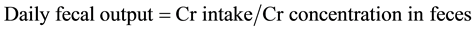 . Forage DM intake was calculated using the equation:
. Forage DM intake was calculated using the equation: 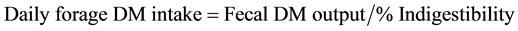 [25] . Intake and fecal excretion of P were then calculated by multiplying forage and fecal concentrations of P by forage DM intake and fecal DM output, respectively.
[25] . Intake and fecal excretion of P were then calculated by multiplying forage and fecal concentrations of P by forage DM intake and fecal DM output, respectively.
2.6. Fecal Pat Degradation
Twenty-four 1-m2 plots were demarcated adjacent to the grazed pasture plots at the research site. These plots were mowed to a 4-cm height and sprayed with glyphosate prior to fecal-pat degradation experiments conducted over a 2-yr period. The study was arranged as a completely randomized design with two replications. Prior to feces collection in the CS and WS, three 20-cm soil samples were taken from each 1-m2 plot and separated into 0 to 5, 5 to 10, and 10 to 20 cm depth strata. In the CS and WS of 2011 (April 21 and September 14, respectively) and 2012 (April 11 and September 12, respectively), feces were collected directly from each animal in each pasture. Each animal was brought to the Stanley P. Wilson Beef Teaching Unit holding facility at 0800 h where feces were collected from a concrete floor immediately following excretion and placed into a bucket. All feces from 0800 to 1500 h from each animal were composited and allocated to 0-, 28-, 56-, 84-, and 112-d after application (DAA) treatments, and a 500-g fecal sample (wet wt) was prepared for each DAA treatment. Zero-DAA fecal samples were taken directly to the laboratory and dried at 60˚C for 72 h. All remaining DAA- treatment fecal aliquots were transported from the holding facility to the experimental plots and randomly placed in the center of the 1-m2 plot. Feces were applied so that a 20-cm diameter fecal pat was constructed in the center of each plot, which ensured that no cross-contamination of nutrients occurred between plots during the trial.
On the assigned treatment DAA, remaining fecal material that had not decomposed was recovered by removing any visible fecal matter remaining on soil from each plot, and subsequently weighed. Three additional soil samples were taken from directly beneath fecal pats and separated into 0 to 5, 5 to 10, and 10 to 20 cm depth strata. Feces and soil were dried at 60˚C for 72 h. Fecal samples were ground with a Wiley Mill (Thomas Scientific, Philadelphia, PA) to pass a 1-mm screen, and all soil samples were sieved to pass a 2-mm screen.
Soil and fecal concentrations of N were determined via dry combustion using a LECO TruSpec CN Analyzer (LECO Corp, St Joseph, MI). Soil samples were extracted using dilute HCl and HNO3 (Mehlich 3), and analyzed by ICAP spectroscopy to determine P (Spectro Ciros CCD, Kleve, Germany) [24] . Concentrations of P in fecal samples were analyzed by dry-ashing followed by ICAP spectroscopy [24] . Fecal samples were also analyzed for water-extractable P using the [26] method. Mean nutrient composition of feces is reported in Table 1. Total remaining nutrients in feces were calculated by the equation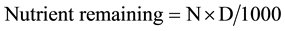 , where N is the concentration of the nutrient and D is the dry weight of the feces on a given DAA.
, where N is the concentration of the nutrient and D is the dry weight of the feces on a given DAA.
2.7. Statistical Analysis
Data were analyzed as a completely randomized design using PROC MIXED of Statistical Analysis Software (SAS, Carey, NC). Fecal nutrient and P utilization data were analyzed using N-fertilization treatment, season and their interaction as main effects. The soil nutrient model included N-fertilization treatment, season, soil depth, DAA and their interactions as main effects. Orthogonal contrasts were used to compare the 0 N treatment
![]()
Table 1. Nutrient composition of feces (dry wt. basis) from cattle grazing pastures receiving different N-fertilization and used in fecal degradation study.
a0 N = 0% N fertilization, 50 N = 50% N fertilization, 100 N = 100% N fertilization based on N requirement of grass species. bCS = cool season and WS = warm season.
with 50 N and 100 N treatments, and 50 N and 100 N treatments. In recognition of the low statistical power characteristic of field studies that employ limited numbers of replicates, α was set to equal 0.10 [27] . Due to lack of a significant year effect, all data presented are pooled across both years.
3. Results and Discussion
3.1. Phosphorus Intake and Fecal Excretion
Phosphorus concentration in forage available to cattle during the experimental period (Table 2) was not different (P > 0.10) between seasons or among treatments. However, forage DM mass was greater (P = 0.008) for CS forage than WS forage, and P mass was greater (P = 0.023) for CS forage than WS forage. Neither DM mass nor forage P mass were different (P > 0.10) among N-fertilization treatments. Foliar P concentration and therefore, forage P mass were affected by soil P status, P fertilization, stage of forage maturity, forage management practices, P apportionment among individual plant species, and meteorological conditions [28] .
Phosphorus intake by cattle (Table 3) was not different (P > 0.10) between grazing seasons or among
![]()
Table 2. Forage DM mass, foliar P concentration, and forage P mass of pastures receiving different N-fertilization treatments.
a0 N = 0% N fertilization, 50 N = 50% N fertilization, 100 N = 100% N fertilization based on N requirement of grass species. bCS = cool season and WS = warm season. cn = 6. d,eWithin a column, means without a common superscript differ (P < 0.10). f,gWithin a column, means without a common superscript differ (P < 0.05).
![]()
Table 3. Phosphorus intake, fecal P output, and fecal water-soluble P output by cattle grazing pastures receiving different N-fertilization treatments.
a0 N = 0% N fertilization, 50 N = 50% N fertilization, 100 N = 100% N fertilization based on N requirement of grass species. bCS = cool season and WS = warm season.cn = 6.
N-fertilization treatments. A treatment × season interaction (P = 0.087) was observed such that fecal P output in CS was greater (P < 0.10) for 100 N than 50 N, but was not different in WS. According to [3] , medium-frame growing beef cattle require approximately 10 to 12 g P/d. In the current study, cattle ingested at least 11.2 g P/d, which is within the recommended intake. Reference [28] has stated that cattle grazing forage typically only become P deficient when soil is deficient in P because foliar P concentration and animal performance are both positively correlated with soil P concentration. Several studies [10] -[12] have reported that fecal P excretion is directly related to P intake, and that a positive correlation exists between increased P in the diet and increased fecal P excretion. Reference [12] has suggested that dietary P intake could be estimated from fecal P excretion. Reference [14] observed that P uptake from high-P soils increased with increasing N-fertilization rate in CS grasses up to a N application rate of 168 kg∙ha−1∙yr−1; in WS, N-fertilization rate did not have as much of an impact, but P uptake increased from 14.2 kg/ha to 18.6 kg/ha. Increased foliar P uptake could explain increased P intake and fecal P excretion observed in the CS of the current study. Fecal concentration of water-soluble P was not different between seasons or among treatments (P > 0.10), but the mean concentration (0.2 g/kg) is similar to values (~0.7 g/kg) reported by [19] for fresh beef cattle feces.
3.2. Fecal Dry Matter Degradation
Percentage of fecal-pat DM remaining (Figure 3) was not different among N-fertilization treatments or between
![]() (a)
(a)![]() (b)
(b)
Figure 3. Dry matter mass remaining (% of initial) in fecal pats from cattle grazing pastures receiving 0 (0 N), 50 (50 N), or 100% (100 N) N-fertilization treatments during the cool season (a) and warm season (b). Vertical bars represent standard error of the mean (n = 6).
seasons. The percentage remaining at 28 DAA was greater (P < 0.037) than at 112 DAA, but was not different (P > 0.10) from 56 and 84 DAA. Percentage remaining at 112 DAA was less (P < 0.037) than at 0, 28 and 56 DAA. Reference [20] reported that cattle manure pats had only been reduced by 15% after 15 weeks. Reference [20] reported that cattle manure applied in the winter had decayed by 25% in 140 d, and in the summer by 46% in 140 d. Other studies have reported that cattle manure decomposed to approximately 50% of initial mass in 80 d [29] . In the current study, fecal pats were reduced by an average of 68.8% in 112 d, which is greater than values reported by [20] , but similar to both [21] [29] .
Percentage of P remaining in fecal pats (Figure 4) was not different (P > 0.10) among treatments or between seasons. Zero DAA had a greater (P < 0.002) percentage remaining than all other DAAs. Additionally, 28 DAA had greater (P < 0.0001) remaining P than 84 and 112 DAA. However, 56, 84 and 112 DAA were not different (P > 0.10). Within the WS, percentage of P remaining at 112 DAA was greater (P = 0.010) in 100 N than 50 N, and greater (P = 0.018) in [50 N + 100 N] than 0 N]. Percentage of water-soluble P remaining (Figure 5) was not different (P > 0.10) among treatments or between seasons. Additionally, there was no difference among DAA (P > 0.10). Within WS, the percentage of water-soluble P remaining at 112 DAA was greater (P = 0.084) in 100 N than 50 N, and 100 N was greater (P = 0.054) than 50 N in the CS at 28 DAA. Whereas mass of total P and water-soluble P remaining in fecal pats were not different among DAA, values decreased over time. Reference [17] reported that P disappearance from fecal pats mirrored disappearance of organic matter of soil-applied manure, consistent with the current study in which values for percentage of DM remaining and percentage of P remaining were similar at every DAA. Reference [20] reported that after 15 wk, 80% of P remained within manure
![]() (a)
(a)![]() (b)
(b)
Figure 4. Phosphorus remaining (% of initial) in fecal pats from cattle grazing pastures receiving 0 (0 N), 50 (50 N), or 100% (100 N) N-fertilization treatments during the cool season (a) and warm season (b). Vertical bars represent standard error of the mean (n = 6).
![]() (a)
(a)![]() (b)
(b)
Figure 5. Water-soluble phosphorus remaining (% of initial) in fecal pats from cattle grazing pastures receiving 0 (0 N), 50 (50 N), or 100% (100 N) N-fertilization treatments during the cool season (a) and warm season (b). Vertical bars represent standard error of the mean (n = 6).
pats; however, the experiment only used a 10-g (DM basis) sample, and disappearance was much less than in the current study for which approximately 74% disappearance of P was observed. Another study stated that cow manure due its large proportion of water-soluble P, can be a long-term source of P that can be transported either by surface water runoff or be leached into the soil [18] . Results of the current study support this concept because almost half of the initial water-soluble P in fecal pats was still remaining in past 112 days later. Additionally, the water-soluble P in the fecal pats increased from 0 DAA to 28 DAA in the CS. Reference [30] states that manure nutrients have a long-term effect on soil due to their slow mineralization by macro- and microorganisms and chemical reactions within the soil and on the surface. Therefore, the increase in water-soluble P could possibly be due to conversion of organic P in the manure into water-soluble P.
Percentage of N remaining in fecal pats (Figure 6) was not different (P > 0.10) among treatments or between seasons. Zero DAA had a greater (P < 0.001) percentage of N remaining than all other DAAs, and 28 DAA had a greater percentage of N remaining than 84 and 112 DAA. However, 56, 84 and 112 DAA were not different (P > 0.10). Reference [17] observed that N loss from fecal pats, much like P, mirrored that of fecal pat DM disappearance; this pattern was observed in the current study, with the greatest N content recorded at 0 DAA and all other subsequent DAA having decreased total N. Additionally, [29] reported that at 87 d the percentage N remaining in surface-applied cattle manure was approximately half of the initial.
Extractable soil P concentration data (Table 4) were extremely variable due to uncontrollable environmental factors (air and soil temperature, moisture, etc.) that precluded our ability to discern any statistical differences
![]() (a)
(a)![]() (b)
(b)
Figure 6. Total nitrogen remaining (% of initial) in fecal pats from cattle grazing pastures receiving 0 (0 N), 50 (50 N), or 100% (100 N) N-fertilization treatments during the cool season (a) and warm season (b). Vertical bars represent standard error of the mean (n = 6).
among the N-fertilization treatments. However, there was a significant season effect such that soil in the WS contained a greater P concentration than in the CS. Also, the 0 to 5 cm depth interval (115.1 mg/kg) had greater (P < 0.001) P concentration than both 5 to 10 and 10 to 20 cm (36.4 and 43.3 mg/kg, respectively); however, 5 to 10 and 10 to 20 cm were not different (P > 0.10). Concentration of soil P on 0 DAA (31.8 mg/kg) was less (P < 0.060) than on both 56 DAA (67.0 mg/kg) and 112 DAA (52.9 mg/kg), but was not different from 28 DAA (98.6 mg/kg) and 84 DAA (74.6 mg/kg). Also, soil on 28 DAA had greater (P = 0.033) soil P concentration than on 56 DAA. Reference [18] reported that P concentration in soil beneath cattle manure had the greatest P concentration from 0 to 2 cm (648 mg/kg), and P concentration decreased until 8 to 10 cm (236 mg/kg). Reference [31] reported soil-available P varied from year to year; however, soil-available P concentrations at the beginning and end of the study were similar and, therefore, no clear trend of available P accumulation following eight annual applications of dairy cattle manure was observed. This observation is in agreement with our finding in the current study that extractable soil P concentration in the CS returned to approximately initial soil P concentration by 112 DAA and, therefore, annual applications during the CS would likely not result in a buildup of extractable soil P. Warm-season soil P concentrations initially increased 10-fold from DAA 0 to 28, which presumably is a result of the greater rate of disappearance of WS fecal pat P than CS fecal pat P. During the time span of the current study, soil P concentrations did not return to initial P concentrations; however, soil P decreased by at least 150 mg/kg. We propose that the dramatic increase of WS soil P concentrations was possibly
![]()
Table 4. Extractable P concentration (mg/kg) in soil from beneath feces from cattle grazing pastures receiving different N-fertilization treatments.
a0 N = 0% N fertilization, 50 N = 50% N fertilization, 100 N = 100% N fertilization based on N requirement of grass species. bDAA = days after application. cn = 6. d,eWithin a row, means without a common superscript differ (P < 0.001).
due to above-average rainfall that occurred in both 2010 and 2011 in August and September (DAA 0 - 56), as opposed to the below-average rainfall that occurred during DAA 0 - 56 in the CS. This additional rainfall would maintain optimum soil moisture levels, allowing for maximum P movement into soil via soil bioactivity.
The total soil N concentration beneath fecal pats did not vary with time or season. The 0N (1.3 g/kg) treatment was not different (P > 0.10) from [50 N + 100 N]; however, 100 N (1.5 g/kg) had a greater (P = 0.018) N concentration than 50 N (1.2 g/kg). Also, total soil N decreased (P < 0.061) as soil depth increased, with 0 to 5 cm depth interval (2.3 g/kg) having the greatest total soil N concentration, 5 to 10 cm having the median value (1.0 g/kg), and 10 to 20 cm having the least value (0.8 g/kg). There was a season × N-fertilization treatment interaction such that 0 N (1.2 g/kg) had less (P = 0.001) total N than [50 N + 100 N] (1.4 g/kg), and 50 N (1.2 g/kg) had less (P = 0.050) total soil N than 100 N (1.7 g/kg) in the CS. Total soil N concentration decreased with increasing soil depth. Lithourgidis et al. (2007) reported similar findings; i.e., that N concentration in soils beneath applied cattle manure did not differ between the beginning and end of the experiment (0- to 30-cm soil depth).
4. Conclusion
In summary, neither N-fertilization regime nor grazing season affected intake or fecal excretion of water-soluble P by grazing cattle, but total P excretion was greater with greater N-fertilization application in the CS. These findings indicated that cattle requirements for P were met and that sufficient P was returned to pasture to meet forage requirement for growth in the absence of fertilization with N. There was no effect of N fertilization on the decomposition of or P removal from fecal pats, or on P concentration in soil beneath fecal pats. However, N fertilization increased N removal from fecal pats and increased total soil N concentrations beneath fecal pats. These observations are interpreted to mean that, in grazed pastures with high soil-test P, N fertilization do not affect intake and fecal returns of P by grazing cattle, foliar uptake of P, and rate or extent of assimilation of P returns into the soil profile from degradation of fecal material.