Performance of Thermal Energy Storage Unit Using Solid Ammoniated Salt (CaCl2-NH3 System) ()
1. Introduction
Recently, the possibility of significant global warming resulting from emissions of greenhouse gases by fossil fuel combustion has become an important concern within the international community. In the energy sector, energy utilization technologies in many industries are expected to develop high efficiency and high performance. For the sake of thermal energy storage systems utilizing the low heat source as solar energy or hot effluent (approx. 353 - 373 K), the processes using the chemical reaction of an anhydrous salt with NH3 have been proposed and discussed for their practicability [1] - [5] . For example, some prototypes of energy storage unit using CaCl2·mNH3 system have been designed and measured these performances and thermophysical properties [3] [5] - [8] .
In this study, the chemical reaction of CaCl2·4NH3 with 4NH3 was chosen here for the thermal energy storage system (see the following chemical reaction: ammoniation and deammoniation), because this reaction system can be driven by using low temperature heat sources, Furthermore, the salt is low cost and easy to supply.
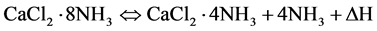
In this reversible chemical reaction, ΔH (enthalpy change) is 43.8 kJ/mol at 0.1 MPa, 304 K [1] , and the value is considerably higher than the latent heat of vaporization of liquid NH3, 23.4 kJ/mol at 0.1 MPa, 240 K [2] . Furthermore, this chemical reaction is well known as higher energy density system as compared with those reactions for other energy storage systems. However, this reaction system is accompanied by a remarkable volume expansion, and the system has an extremely low heat transfer rate of the solid phase where powdered crystal is packed with NH3 gas. In order to improve the low heat transfer rate of the solid phase, a metal fin was inserted in the reactor and the solid phase was suspended in an organic solvent such as 1-heptanol [4] . In authors’ previous work [5] , in the viewpoints of the activation of this chemical reaction on repeated runs and the effect of heat transfer media (Ti: titanium) on the heat transfer of ammoniation, the reaction characteristic of the small vertical reactor unit using CaCl2·mNH3 system was measured in detail, and the effects of weight ratios of Ti on heat flow rate were examined experimentally.
In this study, the prototype of the horizontal energy storage unit was designed for the examination of the effect of the heat transfer media (Ti: titanium) on heat transfer rate of ammoniated salts (CaCl2·mNH3), and performance tests were carried out under the three weight ratios of Ti (Ti/CaCl2 = n, where n = 1, 3, 5). The reaction times required for ammoniations and deammoniations were measured, and the heat flow rates from the ammoniated salt to a brine (water) as the effects of the weight ratios of Ti were measured and compared with that of CaCl2 alone (Ti/CaCl2 = n, where n = 0). Furthermore, the activation of this chemical reaction and the corrosion of this horizontal stainless steel reactor were examined on the repeated runs (≥30 times each).
2. Experimental Section
2.1. Materials
CaCl2 used in this experiment is produced by Wako Pure Chemicals Industries, Ltd. It is guaranteed reagent grade, and it is specified as the pure grade having minimum purity of 95.0% and used without further purification. The powdered crystal of CaCl2 is dried at 773 K and is stored over silica gel in a desiccator. NH3 gas of 99.99% purity is provided from Sumitomo Seika Co. Ltd. Titanium sponge (Ti) of 10 - 28 JIS mesh 90% up is provided from Wako Pure Chemical Industries, Ltd., and it is used as the heat transfer media and has minimum purity of 99.0%.
2.2. Experimental Apparatus
Figure 1 schematically shows the experimental apparatus of the horizontal energy storage unit. This unit consists of horizontal stainless steel reactor, NH3 glass vessel, pressure regulator valve, pressure gauges, thermocouples and constant temperature water baths. The volume of this reactor is approximately 0.8 × 10−3 m3 (its inner diameter is 44.6 mm) and this reactor is covered with the water jacket, and the temperature in this reactor can be controlled. The NH3 vessel is pressure resistant glass vessel, which volume is 0.3 × 10−3 m3, (up to 2.0 MPa), and the volume of liquid NH3 is measured by the microscope with an accuracy of ±0.05% of full volume (0.5 × 10−3 m3).
In order to insulate this reactor from the surroundings, the apparatus is wrapped by the foamed polystyrol. The each temperature in this apparatus is measured by using C-A (Chromel-Alumel) thermocouples corrected by the digital thermometer, and the temperature data as the digital signal (change of mV) is transferred to the microcomputer and stored. The amount of liquid NH3 transferred to the reactor from NH3 vessel can be measured by
![]() 1: Reactor; 2: Water jacket; 3: NH3 glass vessel; 4: Flow meter; 5: Thermocouple;6: Digital thermometer; 7: Microcomputer; 8: Pressure gauge;9: Pressure regulator valve; 10: Needle valve; 11: Constant temperature bath; 12: Pump
1: Reactor; 2: Water jacket; 3: NH3 glass vessel; 4: Flow meter; 5: Thermocouple;6: Digital thermometer; 7: Microcomputer; 8: Pressure gauge;9: Pressure regulator valve; 10: Needle valve; 11: Constant temperature bath; 12: Pump
Figure 1. Schematic diagram of horizontal energy storage unit.
the microscope. The temperatures of this reactor and NH3 vessel are controlled by using the constant temperature water bath throughout the experiment, and the accuracy of temperature control is minimum accuracy within ± 0.1 K. The each pressure in these vessels is measured by Bourdon gauge, which accuracy is ± 0.1 % of full scale (up to 2.0 MPa). The pressure control in the reactor is carried out using the pressure regulator valve.
2.3. Experimental Procedure
CaCl2 of 1 mole (approx. 110 g) is crushed below size of 10 JIS mesh and was dried at 773 K for 3 hours by an oven. A dried CaCl2 sample (or a sample mixed with weighed Ti: weight ratio; Ti/CaCl2 = n, where n = 1, 3, 5) is placed in this reactor. It is sealed, and worked by the vacuum pump in order to remove an air and any water from this system. NH3 vessel is also evacuated for 2 hours and NH3 gas is introduced from the NH3 gas bomb into NH3 vessel, which is kept at a constant temperature (273 K) by the cooling liquid. After liquid NH3 is charged in it, its volume is measured by the microscope rapidly and recorded. Then this reactor is connected with NH3 vessel shown in Figure 1. NH3 gas from NH3 vessel is moved to the reactor through the pressure regulator valve keeping the constant pressure (0.5 MPa) during the reaction. The level of liquid NH3 in the glass vessel is measured by reading the scale of NH3 vessel using the microscope, and the mole number of NH3 absorbed to the dried CaCl2 is calculated from this volume change of liquid NH3 in NH3 vessel. The temperature distribution in detail is measured at the some points of horizontal axis in this reactor. The distance between the measuring point and next one is 0.05 m, and the temperature distribution at 12 points is measured using thermocouples at the same time. The each reaction process in detail is as follows.
2.3.1. lst-Ammoniation and 1st-Deammoniation (CaCl2 ⇒ CaCl2·8NH3 ⇒ CaCl2·4NH3)
When the temperatures of the reactor and NH3 vessel are stabilized, a needle valve is opened to keep the constant pressure using the pressure regulator valve in this reactor. Operating temperature and pressure in the reactor are controlled to 303 K and 0.5 MPa, respectively. The amount of liquid NH3 transferred to the reactor from NH3 vessel is measured by reading the scale of NH3 vessel using the microscope. The NH3 mole number absorbed to CaCl2 is calculated from the volume change of liquid NH3 in NH3 vessel. When 8 moles of NH3 is absorbed to the pure CaCl2, the experiment of 1st-ammoniation is just finished. The 1st-deammoniation from an ammoniated salt (CaCl2·8NH3) is carried out by using the same experimental apparatus. In this case, the NH3 vessel is kept at constant temperature of 293 K by the circulating water from the constant temperature water bath, and the temperatures on horizontal axis in the reactor are heated to 353 K by the heating water. The NH3 mole number desorbed from ammoniated salt is calculated by the same method of 1st-ammoniation. When 4 moles of NH3 is desorbed from CaCl2·8NH3, this 1st-deammoniation process is finished.
2.3.2. Experiment of Repeated Runs (CaCl2·4NH3 ⇔ CaCl2·8NH3)
In order to examine the activation of chemical reaction and the corrosion of this steel reactor, these processes (ammoniation and deammoniation) are repeated alternately (≥30 times each). Experimental conditions are just same as the above two processes. After the final experiment, this horizontal reactor is opened, and the photograph of the ammoniated salt mixed with Ti is taken for observation. The effect of Ti on the repeated cycles is examined, and experimental tests are carried out under the preceding weight ratios of Ti.
2.3.3. Effect of Heat Transfer Media for Heat Flow Rate
In order to discuss the effect of Ti on the performance for ammoniations and deammoniations, the heat flow rates for this reactor are measured. The inlet and outlet temperatures of the circulating water are measured by thermocouples continuously. The flow rate of the circulating water is determined by using the flow meter. The heat flow rate (Q: (kJ/h)) is calculated from the temperature difference (ΔT: Tin − Tout (K)) between inlet and outlet of the circulating water, the flow rate (q: (kg/h)) of the circulating water and the specific heat (Cp: (kJ /(kg∙K)) of the circulating water. Therefore, the heat flow rate is obtained as the following equation, and the heat flow rates are compared under the preceding weight ratios of Ti.
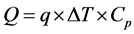
3. Results and Discussion
Figure 2 shows the relation between mole number of NH3 absorbed to CaCl2 and reaction time at constant pressure (0.5 MPa). This figure showed 1st-ammoniation of CaCl2 under the different weight ratios of heat transfer media (Ti). The reaction time required for 1st-ammoniation (CaCl2 + 8NH3 ⇔ CaCl2·8NH3) was decreased with increasing the weight ratio of Ti. These results were similar to the result that we could get from the performance test using the vertical energy storage unit previously [5] .
Furthermore, the effect of Ti on reaction time was effectual over weight ratio of n = 3. Since this chemical reaction is accompanied by a remarkable volume expansion at 1st-ammoniation, it is very important for the performance test that the determination of initial mass of CaCl2 placed in the horizontal reactor. This is because that the space for the volume expansion of CaCl2 with the ammoniation in the reactor is necessary. Then, it was made sure that the apparent volume of ammoniated salts did not decrease after the 1st-deammoniation from CaCl2·8NH3 to CaCl2·4NH3. Therefore, the initial mass of sample was determined by the simple estimation method obtained from previous experiments [5] . Judging from the photograph, the replenishment of CaCl2 in the reactor was in good condition.
Figure 3 shows the relation between mole number and reaction time for 2nd-ammoniation. The reaction time required for 2nd-ammoniation process was shorter than that for 1st-ammoniation. The reaction time required for 2nd-ammoniation process was shorten with increase of weight ratio of Ti. The reaction times required for ammoniations were approximately 16% for n = 1, 41% for n = 3 and 54% for n = 5 shorter than that for CaCl2 alone (n = 0), respectively. It was found that weight ratio over n = 3 was very effectual for the time reduction of each cycle.
![]()
Figure 2. Relation between mole number of NH3 absorbed to CaCl2 and reaction time for 1st-am- moniation.
Figure 4 shows the relation between mole number desorbed from ammoniated salts and reaction time at constant pressure (0.5 MPa). The reaction times required for deammoniations were approximately 19% for n = 1, 50% for n = 3 and 59% for n = 5 shorter than that for CaCl2 alone (n = 0), respectively. These values were similar to that for ammoniations. Weight ratio over n = 3 was exceedingly effectual for the time reduction of deammoniation. The similar results were obtained on repeated runs, and the decrease of activation for this chemical reaction could not be observed in these experimental trials (≥30 times each).
Figure 5 shows the effect of heat transfer media on heat flow rate (Q). When Ti was mixed with pure CaCl2
![]()
Figure 3. Relation between mole number of NH3 absorbed to CaCl2·4NH3 and reaction time.
![]()
Figure 4. Relation between mole number of NH3 desorbed from CaCl2·8NH3 and reaction time.
![]()
Figure 5. Effect of heat transfer media on heat trans- fer rate.
at weight ratio of n = 1, heat flow rate did not change approximately. The heat flow rate was only 10% increase in comparison with that for CaCl2 alone. On the other hand, those values for weight ratio of n = 3 and n = 5 were greatly changed, these values of heat flow rates were approximately 50% and 60% larger than that of CaCl2 alone, respectively. These results meant that the heat flow rate per hour became high value with reducing of reaction times required for ammoniations. It was thought that the ratio over n = 3 was the most effective as the amount of Ti in these experiments.
4. Conclusion
In order to improve the energy storage unit using CaCl2·mNH3 system, Ti (titanium sponge) as a heat transfer media was mixed with CaCl2 under the conditions of 3 weight ratios. With increasing the weight ratio of Ti, the reaction times required for ammoniations and deammoniations were approximately 16% - 59% shorter than that for CaCl2 alone. On the other side, the heat loss increased with increasing the weight ratio of Ti. However, the addition of Ti was very valuable for the improvement of heat transfer rate of gas-solid reaction. Furthermore, these similar results were obtained during the repeated runs of ammoniations and deammoniations. The decrease of activation of chemical reaction and the corrosion of the stainless steel reactor could not be observed in these experimental trials (≥30 repeated runs). The recycle of Ti was an easy operation. These values of heat transfer rates for the ammoniations of CaCl2 mixed with Ti (weight ratio; Ti/CaCl2 = 3, 5) using the horizontal energy storage unit were approximately 50% and 60% larger than that of CaCl2 alone, respectively. These results indicate that the adding the heat transfer media to ammoniated salts such as CaCl2·mNH3 system is available for the improvement of other gas-solid reactions.
NOTES
*Corresponding author.