1. Introduction
Hybanthus enneaspermus L. Muell., (Syn. Ionidium enneaspermus DC) is a perennial ethanobotanical herb and it belongs to the family Violaceae. It grows 15 - 30 cm in height with many diffuse or ascending branches and is pubescent in nature [1]. This ethnobotanical herb is known to have unique medicinal properties. Traditionally, H. enneaspermus is used as an aphrodisiac, demulcent, tonic, diuretic, in urinary infections, diarrhoea, leucorrhoea, dysuria, and sterility [2]. The fruits and leaves are used as antidotes for scorpion stings and cobra bites by the Yanadi tribes [3,4]. The growth of Plasmodium falciparum resistant K1 strain was best inhibited by methylene chloride extract of H. enneaspermus [5]. The roots of H. enneaspermus possess marked nephroprotective activity and could have promising role in the treatment of acute renal injury [6].
In view of this ethanomedicinal medicinal property, H. enneaspermus plant has drawn a massive attention for mass propagation. The natural regeneration potential of this herb is very poor due to low seed viability [7]. Plant tissue culture is a gateway for conservation and mass propagation of rare and endangered medicinal plants. Though, in vitro micro propagation from seed-derived callus was reported by Prakash et al., 1999 and achieved only 8.9 shoot, high frequency direct shoot induction in H. ennesaspermus has not yet been reported. Hence an efficient and simple protocol for high frequency direct shoot induction for mass propagation has been developed and reported here.
2. Materials and Methods
2.1. Plant Material
Young leaves (0.5 - 1 cm) were collected from 2 month old disease free healthy H. enneaspermus plants grown in the Botanical garden of V.H.N.S.N College, Virudhunagar, Tamilnadu, India and used as explants.
2.2. Prepation of Explants
The explants were washed in running tap water for 30 minutes and then thoroughly rinsed in detergent solution (Tween 20) for 3 minutes. They were then surface disinfected with HgCl2 (0.1%) for 1.0 minute and thoroughly washed with sterile distilled water.
2.3. In-Vitro Culture
Explants were inoculated on MS (Murashige and Skoog 1962) basal medium supplemented with three percent sucrose, kinetin (KI; 2.32, 4.64, 6.96 and 9.29 µM), 6-benzylaminopurine (BAP; 2.22, 4.44, 6.66 and 8.88 µM) either individually or in combination of KI and BAP for shoot bud induction.
For enhancing the shoot induction, various auxins like 3-Indoleacetic acid (IAA; 2.85, 5.71, 8.56 and 11.42 µM) or 3-Indolebutyric acid (IBA; 2.46, 4.92, 7.38 and 9.84 µM) were tested along with 2.32 µM KI and 2.22 µM BAP. The effect of coconut milk (CM 10%) and adenine sulphate (Ads 370.94 µM) were also tested either individually or in combination on MS medium fortified with 2.22 µM BAP and 2.32 µM KI with auxin (IAA) or without auxin (IAA) for shoot induction.
All media contained 0.8% (w/v) agar. The pH of the medium was adjusted to 5.8 using 0.1N NaOH or 0.1N HCl before autoclaving at 121˚C for 15 min. The cultures were incubated at 25˚C ± 2˚C and 70% - 80% relative humidity under a 16-hrs photoperiod.
The regenerated shoots (2 to 3cm) were rooted on half strength MS medium supplemented with various concentrations of 3—Indolebutyric acid (IBA; 2.46, 4.92, 7.38, 9.84 µM) or α—Naphthaleneacetic acid (NAA; 2.68, 5.37, 8.05, 10.74 µM). After roots were developed, the plantlets were transplanted to pots filled with vermiculate and sand (1:1 ratio) and kept in growth chamber with 70% - 80% humidity under 16hrs photoperiod. After acclimatization, the plantlets were transferred to the garden and survival percentage was calculated. Table 1 show Plant Growth Regulators and other supplements tried in cultures of H. enneaspermus.
2.4. Statistics
Single explant was used per flask. Each experiment was repeated at least thrice using triplicate. Data were statistically analyzed and means were compared using Duncan’s multiple range test (P ≤ 0.05).

Table 1. Plant growth regulators and other supplements tried in cultures of H. enneaspermus.
3. Results and Discussion
Callus initiation was observed from the cut ends of the explants after seven days and shoot organogenesis was observed within 15 days of culture. Multiple shoots were formed followed by callus formation on MS medium fortified with different concentration of KI (2.32, 4.64, 6.96 and 9.29 µM·l–1), BAP (2.22, 4.44, 6.66, 8.88 µM·l–1) and in combination of KI and BAP (KI 2.32 µM·l–1, 4.64 µM·l–1, BAP 2.22 µM·l–1, 4.44 µM·l–1) (Table 2). The callus first appeared light-yellow in colour and friable in texture then became dark green and compact callus. Highest shoot induction as well as average number of shoot was observed when the medium was supplemented with 2.32 µM·l–1 KI and 2.22 µM·l–1 BAP. Shoot formation was less when the medium was containing KI or BAP alone. Among KI or BAP on medium, KI has better performance compared to BAP in view of shoot induction. Higher concentration of either KI or BAP on culture medium had negative effective on shoot bud induction. This report finds the synergistic effect of BAP and KI on multiple shoot induction. The role of BAP or KI alone on MS medium for shoot bud induction was reported in many plants [8]. In contrast, high frequency multiple shoot induction by the combination of BAP and KI reported in few [9]. Banerjee and Shrivastava [10] also reported the pooled effect of BAP
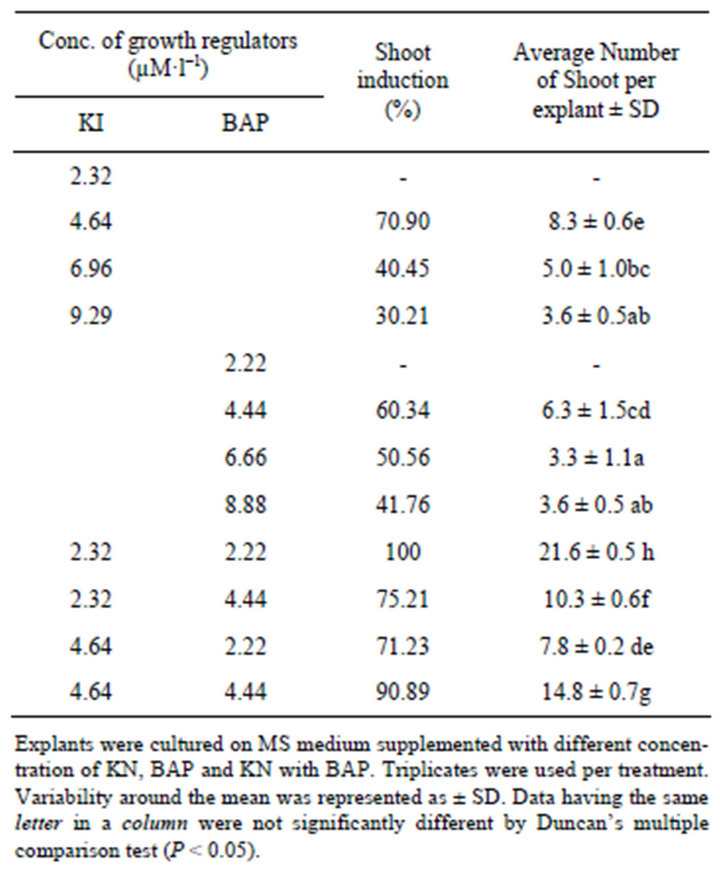
Table 2. Effect of cytokinins on direct shoot induction in Hybanthus enneaspermus (L.) F. Muell (after 4 weeks).
and KI in in-vitro multiplication of Bacopa monnieri.
To enhance the shoot induction, various auxins (IAA and IBA) were used along with optimized concentration of 2.32 µM·l–1 KI and 2.22 µM·l–1 BAP. When auxin was used along with KI + BAP numerous shoot buds were induced, in addition to the callus at the cut end of the explants. The degree of callus formation increases with the increase in the concentration of auxin. Explants showed high frequency of shoot induction and decreased callus formation on medium containing IAA with 2.32 µM·l–1 KI and 2.22 µM·l–1 BAP. Shoot induction and average number of shoots (52.3 ± 1.5) from the single explant was highest when the medium is supplemented with 5.71 µM·l–1 of IAA along with cytokinins (2.32 µM·l–1 KI + 2.22 µM·l–1 BAP) at 4-wks culture. Though IBA promoted the shoot induction, the number of shoots produced was more in IAA (Table 3).
The Coconut Milk (CM) and Adenine Sulphate (Ads) have no effect on enhancing shoot induction (data not shown). Rout [11] also reported that KI in the presence of Ads or Ads and IAA did not promote shoot bud regeneration and direct plant regeneration of Murraya koenigii.
Regenerated shoots were classified into two groups based on their morphological appearance. Explants cultured on MS medium supplemented with BAP alone formed cylindrical stems with 5 - 6 cuneate leaves and appeared as normal (Figure 1(c)). However explants cultured on MS medium supplemented with KI and BAP formed thin stem and small leaves (Figure 1(d)). Mandal [12] reported three groups regenerated shoots on regeneration system of safflower and pointed that low concentration of BAP favoured the induction of normal shoots. Ziv [13] also reported about the effect of BAP in promoting hyperhydric shoots in several instances.

Table 3. Effect of auxin on direct shoot induction in Hybanthus enneaspermus L. Muell (after 4 weeks).
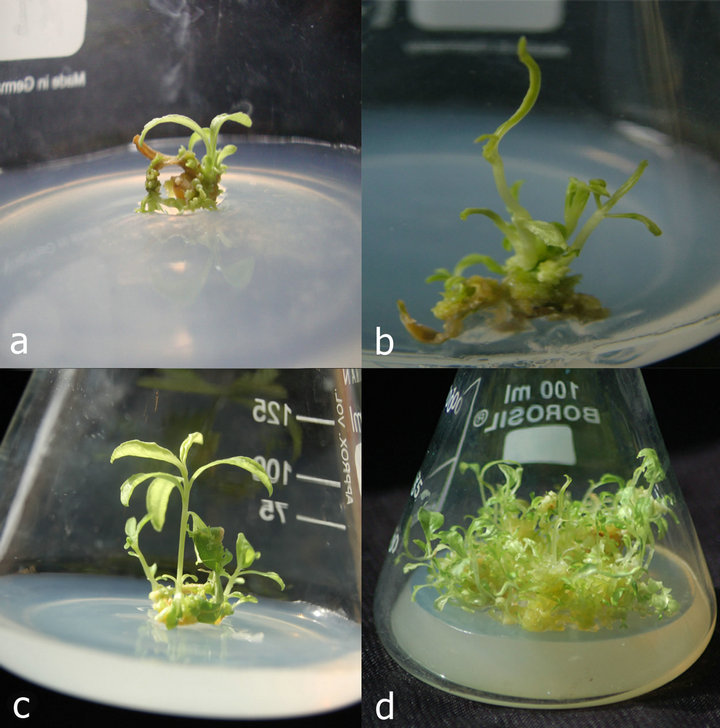
Figure 1. (a) Callus initiated from leaf explants of H. enneaspermus along with the induction of shoot on MS medium with 2.32 µM·l–1 KI after 15 days; (b) Direct shoot with high degree of callusing formed from leaf explants in the presence of high auxin; (c) Direct shoots formed with normal cuneate leaves from leaf explants at 25 days on MS medium with 4.44 µM·l–1 BAP d) Direct shoots formed with thin stem and small leaves from leaf explant at 40 days on MS medium with 2.32 µM·l–1 KI and 2.22 µM·l–1 BAP.

Table 4. Effect of auxin on root induction in Hybanthus enneaspermus L. Muell (after 4 weeks).
For root initiation, morphologically normal shoots (1.0 to 1.5 cm in length) were transferred to half strength MS medium supplemented with growth regulators. The regenerated shoots (1.0 - 1.5 cm in length) were rooted on half-strength MS medium supplemented with various concentrations IBA (2.46, 4.92, 7.38, 9.84 µM) or NAA (2.68, 5.37, 8.05, 10.74 µM). In most of the study, only reduced strength of the basal medium was used for rooting of adventitious shoots and here also reduced strength of basal medium was employed in this study. Roots started to emerge from the cut end of the shoots within 20 days of transfer to rooting medium. The highest frequency of root initiation (100%) with maximum number of roots (21 per shoot) after 4 wks cultures was achieved on a half strength MS basal medium supplemented with 2.46 µM·l–1 IBA (Table 4). NAA also yield significant number of shoots after 4 wks cultures. Roots initiated with IAA and IBA were found to be thin and weak in Decalepis arayalpathra [14].
According to Kirdmaee [15] the success of invitro propagation can be effectively evaluated by the number of plantlets that are successfully transferred to field condition. The rooted shoots did not require any special treatment in the growth chamber for transplantation. The rooted shoots were kept in soil along with vermiculate (1:1 ratio) in growth chamber with 70% - 80% humidity under 16hrs photoperiod. More than 90% of the plants survived in the field. They grew well and have no morphological difference.
In conclusion, this study provides a simple protocol to regenerate high frequency multiple shoot from single leaf explant on MS medium supplemented with 2.32 µM·l–1 KI and 2.22 µM·l–1 BAP with 5.71 µM·l–1 IAA.
NOTES