Influence of Chitosan Binder on the Adhesion of Silver Nanoparticles on Cotton Fabric and Evaluation of Antibacterial Activity ()
1. Introduction
The incorporation of silver nanoparticles (AgNPs) into polymers and/or inorganic carriers is of great interest for many researchers because of the potential applications of these nanocomposite materials in medicine [1] -[5] , photocatalysis [6] [7] , water treatment [8] -[12] , textile [13] -[18] , etc. More details of the immobilization and practical applications of antibacterial nanoparticles on different carriers can be referred to the paper reviewed recently by Moritz and Geszke-Moritz [19] . It is well known that AgNPs have a broad antibacterial activity while exhibiting low toxicity towards mammalian cells at bacterial killing doses [20] [21] . Nanotechnology has facilitated the production of smaller size of AgNPs with the increase of large surface area-to-volume ratios. It is generally accepted that the smaller the AgNPs size, the stronger the antimicrobial activity [22] [23] .
Various methods for the synthesis of AgNPs based on bottom-up approach, i.e. reduction of Ag+ ions to Ago (zero-valent silver) in solution have been reported [24] [25] and the most common method is chemical reduction of silver salt precursor using chemical reducing agents [19] . In comparison with other methods, gamma Co-60 ray irradiation has been considered as an effective method with several advantages as described in our previous paper [24] . Chitosan is a natural polysaccharide derived from the deacetylation of chitin with both antibacterial property and biocompatibility [26] . Chitosan has been used as stabilizer in the synthesis of AgNPs by g-irradia- tion method [25] [27] [28] . AgNPs were stabilized by chitosan through simultaneously steric and electrostatic effect by interaction of -OH and -NH2 groups on the surface of AgNPs. Furthermore, chitosan and AgNPs acted synergistically against bacteria and as a result the AgNPs/chitosan exhibited higher antibacterial activity than any component acting alone [28] . On the other hand, chemical interactions between chitosan and cellulose were reported in chitosan-treated cellulose by diffuse reflectance spectroscopic techniques (UV-Vis and FTIR) [29] . Therefore, chitosan is considered as a suitable binder for the adhesive enhancement of AgNPs with cotton fabric and as an inducer for the synergistic antibacterial activity together with AgNPs for AgNPs/chitosan treated cotton fabric.
In the present study, AgNPs were synthesized by g-irradiation using chitosan as both stabilizer and hydroxyl free radical scavenger and the as-synthesized AgNPs stabilized by chitosan were incorporated onto cotton fabric. The influence of chitosan binder on the adhesion of AgNPs on cotton fabric after repeated washing, the antimicrobial activity against Staphylococcus aureus (S. aureus) and the mechanical property of the as-prepared AgNPs/ cotton fabrics were also investigated.
2. Experimental
2.1. Materials
Analytical grade AgNO3 and lactic acid were purchased from Shanghai Chemical Reagent Co., China. Chitosan made from shrimp shell with deacetylation degree of about 90% and Mw of 9.2 × 104 g/mol was supplied by Chitosan Co., Vung tau, Vietnam. Bacterium strain namely S. aureus ATCC 6538 was provided by University of Medicine-Pharmacy, Ho Chi Minh City. The Mueller Hinton agar medium for bacterial incubation was purchased from Himedia, Mumbai, India. Distilled water was used in all experiments. Cotton fabric weighting 120 g/m2 was provided by VICOTEX Company, Vietnam.
2.2. Methods
2.2.1. Synthesis of AgNPs/chitosan by g-irradiation
Stock chitosan solution (2%, w/v) was prepared by dissolving 2 g chitosan in 100 mL lactic acid solution 1% (v/v) and stored overnight. Chitosan solution was filtered through stainless steel net (200 mesh) to separate undissolved solid. The desired content of AgNO3 was mixed with chitosan solution to prepare three solutions with 2 mM Ag+ and different chitosan concentration of 0.5%, 1% and 2% (w/v). And then, the prepared Ag+/chitosan solutions were put into glass bottles with plastic caps. The irradiation of Ag+/chitosan solutions to prepare AgNPs was carried out on a gamma Co-60 irradiator STSVCo-60/B (Hungary) at VINAGAMMA Center, Ho Chi Minh City with absorbed dose of about 7 kGy [27] [30] .
2.2.2. AgNPs characterization
UV-Vis spectra of AgNPs/chitosan solutions which were diluted by water to 0.2 mM were recorded on an UV-2401PC, Shimadzu, Japan and the size of AgNPs was calculated from TEM images taken on a JEM 1010, JEOL, Japan [27] .
2.2.3. Incorporation of AgNPs onto Cotton fabric and characterization
Before use, cotton fabric was washed to remove glue then dried and cut into equal-sized square pieces of 0.2 × 0.2 m2. All cotton fabric samples were padded in AgNPs/chitosan solutions of about 5 min. and then squeezed to wet pick-up of 100%. Afterwards, AgNPs treated cotton fabrics (AgNPs/cotton fabric) were air dried under ambient conditions. The silver content in AgNPs/cotton fabric samples was determined by inductively coupled plasma- atomic emission spectroscopy (ICP-AES) on a Perkin-Elmer, Optima 5300 DV. The mechanical property (tensile strength, Fb and elongation at break, eb) of AgNPs/cotton fabrics was measured on a Tensile tester Zwick/Roell, Germany following an ASTM method D 5035.
2.2.4. Washing and silver release from AgNPs Incorporated Cotton fabrics
The washing process of AgNPs/cotton fabrics was carried out by the process as described by El-Rafie et al. with 5, 10 and 20 washings [17] . The silver content in AgNPs/cotton fabric after washing was also determined by ICP-AES method.
2.2.5. Antibacterial Activity tests
The antibacterial activity of cotton/AgNPs fabrics was tested against S. aureus by using a shaking flask as described by Zhang et al. [16] with some modifications. Briefly, 1 g sample fabric, cut into small pieces with a size of about 0.25 × 0.25 cm2 was dipped into a flask containing 100 ml of S. aureus suspension with a cell concentration of about 106 CFU/ml. The flask was then shaken at 150 rpm on a rotary shaker at room temperature for 24 h. Afterwards, the number of bacteria forming units (CFU) in each mixture sample was quantified by spread plate on Mueller Hinton agar plates and the antibacterial efficiency, η(%) was calculated as follows [16] [27] :
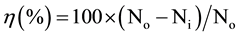
where No and Ni were the CFU/ml from the original cotton fabric and the AgNPs/cotton fabric, respectively.
3. Results and Discussion
3.1. AgNPs/Chitosan characterization
The chitosan stabilized AgNPs solutions with suitably appropriate concentration are protected from aggregation due to both steric and electrostatic stabilization effect of chitosan which has abandon of -OH and -NH2 groups along the molecular chains [25] [31] . Therefore, colloidal AgNPs/chitosan solution is fairly stable during storage time at ambient temperature [27] . The schematic capping mechanism of AgNPs by chitosan has been proposed by Huang et al. [25] . However, they used rather high Ag+ concentration (~40 mM), therefore it caused gelation of Ag+ with chitosan during preparation of Ag+/chitosan solution. In our preparation of 2 mM Ag+ in 0.5% - 2% chitosan solution, no gelation occurred. The synthesis of AgNPs by gamma Co-60 irradiation method, chitosan has been used as a stabilizing and free radial •OH scavenging agent [18] [25] [27] . During irradiation Ag+ ion is reduced to Ago atom by 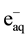 and H• and Ago atoms will be agglomerated to form AgNPs that are capped by chitosan or by other stabilizers. The detail reducing mechanism of formation of AgNPs by gamma Co-60 irradiation method can be referred to the papers reported by Du et al. [24] and Huang et al. [25] . The UV-Vis spectra of 2 mM AgNPs stabilized by 0.5%, 1% and 2% chitosan and TEM images with particle size distribution were shown in Figure 1 and Figure 2, respectively. Table 1 summarized the value of optical density (OD), maximum absorption wavelength (lmax) and average diameter (d) of AgNPs synthesized in different chitosan concentrations.
and H• and Ago atoms will be agglomerated to form AgNPs that are capped by chitosan or by other stabilizers. The detail reducing mechanism of formation of AgNPs by gamma Co-60 irradiation method can be referred to the papers reported by Du et al. [24] and Huang et al. [25] . The UV-Vis spectra of 2 mM AgNPs stabilized by 0.5%, 1% and 2% chitosan and TEM images with particle size distribution were shown in Figure 1 and Figure 2, respectively. Table 1 summarized the value of optical density (OD), maximum absorption wavelength (lmax) and average diameter (d) of AgNPs synthesized in different chitosan concentrations.
The obtained results indicated that the AgNPs diameter for three chitosan (CTS) concentrations namely 0.5, 1 and 2% was not much different from each other in the range of ~7 - 9 nm. The reason may be due to chitosan was used for stabilization of 2 mM AgNPs with high concentration and already reached to the critical concentration of stabilizer for protecting AgNPs to form the smallest particles size. Du et al. already reported the critical concentration of polyvinyl alcohol for preparation of the smallest size (~10 nm) of 20 mM AgNPs by gamma Co-60 irradiation was of 2% - 4% [32] .
![]()
Figure 1. UV-Vis spectra of colloidal AgNPs solutions stabilized with (a) 0.5, (b) 1 and (c) 2% chitosan.
![]()
![]()
Figure 2. TEM images and particle size distribution histograms of AgNPs stabilized in CTS: 0.5 (A,a), 1 (B,b) and 2% (C,c).
![]()
Table 1. Value of OD, λmax and diameter of AgNPs prepared in different chitosan concentrations.
3.2. Silver release from AgNPs/Cotton Fabric by washing
Figure 3 showed the results of silver release from AgNPs/cotton fabric by washings. The obtained results indicated that the suitable concentration of chitosan for better adhesion of AgNPs on cotton fabric was of 0.5% - 1%. The content of silver release from AgNPs stabilized by 0.5 and 1% chitosan after 20 washing cycles was of ~30% compared to that of ~44% for 2% chitosan sample. It indicated that the higher the binding concentration did not result better adhesion of AgNPs on cotton fabric, particularly in case of chitosan. The reason may be due to excessive chitosan content that could not strongly adhere to cotton fibers, therefore during washing, the excessive portion of chitosan will be easily released and taken AgNPs together with. Further study should be carried out to clarify this phenomenon.
3.3. Antibacterial efficiency of AgNPs Coated Cotton fabrics
The antibacterial efficiency of AgNPs/cotton fabrics with different content of AgNPs against S. aureus was presented in Table 2 and Figure 4. It can be observed that all the AgNPs/cotton fabrics with silver content from 124 mg/kg to 245 mg/kg fabric showed highly antibacterial efficiency (>98% compared with untreated cotton fabric). The antibacterial efficiency increased slightly from 98.04% to 99.98% with the increase of the silver content of AgNPs/cotton fabric. According to the results reported by Zhang et al. [16] , the antimicrobial efficiency of AgNPs coated cotton fabric with silver content of about more than 158 mg/kg fabric against S. aureus was almost to reach to h » 100%. The reason of the difference of antibacterial efficiency of Zhang et al. [16] and our result in this work may be due to the cell concentration of S. aureus that they used for antibacterial test was of about 106 CFU/ml which was smaller compared with 107 CFU/ml in our antibacterial test experiment. The results also indicated that after 20 washing cycles, AgNPs/cotton fabric still maintained highly antibacterial activity.
![]()
Figure 3. Silver content of AgNPs/cotton fabrics after washing.
![]()
Table 2. Silver content and antibacterial efficiency against S. aureus of AgNPs/cotton fabrics.
(*) not detected by ICP-AES (untreated cotton fabric).
![]()
Figure 4. S. aureus colonies forming on agar plates: (a) control (cotton fabric); (b), (c), (d) and (e) AgNPs/cotton fabric with 245, 204, 177 and 124 mg silver/kg fabric, respectively.
![]()
Table 3. The value of Fb) and eb of cotton and cotton/AgNPs fabrics.
In addition, according to our results reported in a previous paper, AgNPs/cotton fabrics are innoxious to skin with coefficient of k = 0 [18] . Furthermore, concerning environmental impact of AgNPs, it is also worth to note that the AgNPs in wastewater is almost completely transformed into Ag2S that has extremely low solubility and exhibits a much lower toxicity than other forms of silver [33] [34] . Therefore, AgNPs release from AgNPs/cot- ton fabric into wastewater by washing will be transformed into Ag2S that is considered to have no significant impact to the environment [33] . Therefore, AgNPs/cotton fabric with highly antibacterial activity can be potentially used as bed drapes and/or patient uniforms in hospitals, especially for patients with infectious diseases, etc.
3.4. Mechanical property of AgNPs Coated Cotton fabric
Table 3 presented the mechanical property particularly tensile strength (Fb) and elongation at break (eb) of cotton and AgNPs/cotton fabrics. As a result, Fb and eb of AgNPs incorporated cotton fabrics were almost unchanged in comparison with untreated cotton fabric.
4. Conclusion
Colloidal AgNPs solution was successfully synthesized by gamma Co-60 irradiation method using chitosan as stabilizer and hydroxyl free radical scavenger. The diameter of 2 mM AgNPs stabilized with 0.5% - 2% chitosan was of 7 - 9 nm. AgNPs stabilized with 0.5% - 1% chitosan were found to be better adhesion on cotton fabric. Thus, the as-prepared AgNPs/cotton fabric with highly antibacterial activity, low content of silver release by washing and safety can be potentially used as bed drapes and/or patient uniforms in hospitals, etc. Pilot scale production line with 30 - 50 m2/h of AgNPs/cotton fabric by padding method has been carrying out.
Acknowledgements
The authors are thankful to VINAGAMMA Center, VINATOM for favorable conditions to perform this research. We are also grateful to the contribution of Quy, H.T.D., Van, H.T.H., Diem, P.H.N. and Hoa, T.T. in characterization of materials.
NOTES
*Corresponding author.