Improved High Performance Recycling of Polymers by Means of Bi-Exponential Analysis of Their Fluorescence Lifetimes ()
1. Introduction
There is an increasing need for the recycling of organic polymers (plastics) both for economy and avoiding environmental pollution. The pacific trash vortex is one of the most prominent and impressive examples for the latter and even gets the name Great Pacific Garbage Patch. The majority of technical polymers are thermoplasts and may be re-used by melt and moulding again. However, the immiscibility and the incompatibility of different organic polymers are therefore the main obstacle, because high performance materials require uniformity where a content of external material as low as 5% downgrades the value of polymers appreciably. As a consequence, pure polymers are required for an efficient recycling where a sorting becomes necessary for mixed collected materials. Sorting is also of interest for chemical processing of polymers because such processes operate most stable with uniform starting materials. The machine-based recognition of polymers is a prerequisite for such processes where methods using the density or electrostatic properties are described [1] - [3] . Optical methods [4] [5] are more attractive because of simple, stable and efficient technology where fluorescence is advantageous [6] - [10] because of unproblematic light path and detection. The doping of polymers with fluorescent markers [10] and their re-identification by the spectral resolution of their fluorescence in combination with a binary coding is described in preceding papers [11] [12] , however, a secure pre-treatment of the polymers is a prerequisite for such methods. The use of the remarkably strong autofluorescence of polymers [13] is an attractive alternative because no pre-treatment is necessary and even the workup of deposited material will become possible.
2. Materials and Methods
Spectroscopy: Fluorescence spectra: Varian Cary Eclipse; fluorescence lifetimes: PicoQuant FluoTime 300; Pico Quant PicoHarp 300 (PC-405 laser; 403 nm).
The preferred wavelengths for the detection of the fluorescence decays were located by means of lifetime dependant fluorescence spectra. Starting at the maximum of the fluorescence intensity I(t) with t = 0 and a delay of 3 ns the fluorescence spectrum was recorded with sampling until 100 ns. The maximum λmax of this fluorescence spectrum was used for the determination of the fluorescence lifetimes τ because of an amplification of slower component of decay (the spectrum of the shorter period between 0 and 3 ns was recorded for comparison). The fluorescence intensities were accumulated with a repetition rate of 20 MHz during 10 s; slightly increased noise is obtained for a shortened accumulation time of 1.0 ms. The fluorescence lifetimes were obtained by exponential fitting (exponential tail fit) of the respective sections according to the equation I(t) = A ・ e−t/τ by means of the software FluoFit from PicoQuant. The shape of the laser pulse was neglected due to its small FWHM. The decay time τ1 was calculated from the period between 0 and 3 ns and the decay time τ2 between 3 and 43 ns. Thus, all values are relative and are not independent on the used analytical equipment.
Materials: The technical polymers Luran® (styrene, polyacrylonitile copolymer from BASF), Delrin® (polyoxymethylene from DuPont) and Ultramid® (polyamide with glass fibre from BASF) were applied without further treatment. Spectroscopic grade solvents were applied. Tectosil® from Wacker Chemie AG; PET (polyethylene terephthalate) flakes from Inter Recycling GmbH, applied without further purification.
Purification of PET: PET-Flakes were treated with stirring with a mixture of 3% aqueous NaOH (100 mL) and 15% aqueous sodiumdodecylsulphate solution (SDS, 50 mL) at 85˚C for 2 h, washed with distilled water, dried in air at room temperature and then at 60˚C for 16 h.
3. Results and Discussion
3.1. First Order Decay of Autofluorescence
The spectra of the autofluorescence of polymers are obtained with optical excitation in the UV, such as with common light sources at 365 nm or at short wavelengths in the visible region such as with well-available lasers at 403 nm. The majority of the fluorescence emission extends in the visible at short wavelengths. The fluorescence decay can be mainly described with a first-order exponential function and the lifetime τ can be precisely determined and is characteristic for the polymeric material [13] . The special advantage of first order is the independence of the time constant as well from the initial intensity as the starting time of the measurement. The thus obtained time constants are reported for typical technical polymers in Figure 1.
Here we see that there is a broad range of τ between 0.2 until 5 ns where the individual time constants are as characteristic as fingerprints. Remarkably, the chemically very similar and otherwise difficult to discriminate types of polyethylene, LDPE (low-density polyethylene), HDPE (high-density polyethylene) and UHDPE (ultra- high-density polyethylene) can be unambiguously distinguished; this may be a consequence of different microscopic rigidities of these materials. Moreover, different methods of manufacturing such as PET for bottles and for PET plates or different types of silicones can be well categorized; see Figure 1. The various types of silicone dehesives result in well distinguishable, however similar lifetimes τ. The availability of a further criterion for characterization would be of interest.
![]()
Figure 1. Fluorescence lifetimes τ in ns of technical polymers. Reproducibility is given within 1%.
3.2. Bi-Exponential Components of Fluorescence Decay
We found that the mono-exponential decay dominates resulting in the quite well acceptable first order kinetics. However, there is a bi-exponential component in the decay process. Thus, the lifetimes τ from Figure 1 mean an averaging of both components depending on their relative contributions. We analyzed the fluorescence decay bi-exponentially and additionally obtained the two time constants τ1 and τ2. All three time constants τ, τ1 and τ2 are reported in Table 1.
The two time constants τ1 and τ2 of the bi-exponential data processing allow the two-dimensional characterization of the polymers as is shown in Figure 2; Luranâ (No. 16) is omitted for clearness because recording the very high value of τ1 would compress the abscissa.
The two-dimensional presentation of the fluorescence lifetimes τ1 and τ2 in Figure 2 allows a clear distinguishing even for similar materials and supplements the τ values of Figure 1. It becomes simpler to distinguish as well between the widely applied polyethylenes LDPE, HDPE and UHDPE (filled circles) as differently processed materials such as the silicone elastomer Tectosilâ (triangles) and PET (squares). The differently processed silicone dehesives (diamonds) become more clearly separated where the catalyst for cross-linking (Sn or Pt) seems to influence predominantly τ2, whereas the addition of a hardener (Pt (50)) seems to increase τ1. This corresponds to the very low values of τ1 for the elastomer Tectosilâ.
3.3. Test for Contaminated PET
PET is widely applied both for foodstuff such as bottles for water and soft drinks and for technical liquids such as the mineral oils diesel or engine oil. The latter lipophilic liquids can diffuse into the polymeric material such as a plasticizer and would be slowly released. As a consequence, PET for foodstuff must be carefully separated from the latter material. We simulated such contaminations by the contact with the mineral oils diesel and engine oil for one week and purified PET with alkaline detergents according to a technical standard procedure (see materials and methods).
Figure 3 clearly indicates that a pre-treatment of PET results in a lowering of the fluorescence lifetime τ. This is most pronounced for diesel with low molecular weight and low viscosity and slightly less pronounced for engine oil with higher molecular weight and viscosity; this corresponds to observations with silicone dehesives and Tectosilâ (see Figure 2). A technical cleaning of the contaminated PET increases the fluorescence lifetime again, however, it does not reach the high value of the neat material by far. Even clean commercial recycling flakes exhibit a slightly diminished fluorescence lifetime.
The detection of contaminations becomes more extended by means of the two-dimensional presentation of the fluorescence lifetimes τ1 and τ2 in Figure 4. The recycling flake with τ close to the neat material given in Figure 3 becomes much more separated by τ1 and τ2 in Figure 4. The alterations by means of purifying exhibit different effects for diesel and engine oil. As a consequence, neat material can be well separated.
3.4. Further Extensions
Additional optical information about the polymers could be redundantly used for independent characterization of polymers in order to further increase of the reliability or for fine-tuning of the detection concerning the identifi-
![]()
Figure 2. Two-dimensional characterization of polymers by means of their constants τ1 and τ2 of bi-exponential data processing of fluorescence decay. Filled circles: The polyethylenes LDPE, HDPE and UHDPE; squares: PET; diamonds: Silicones; triangles: The silicone elastomer Tectosilâ.
![]()
Figure 3. Fluorescence lifetimes τ of pre-treated PET; expanded range.
![]()
Figure 4. Two-dimensional characterization of PET with various pre-treatment by means of their constants τ1 and τ2 of bi-exponential data processing of fluorescence decay.
![]()
Table 1. Time constants for the autofluorescence decay of technical polymers. For mono-exponential data processing τ and for bi-exponential τ1 and τ2.
a) PET bottle for soft drinks.
cation of certain batches. The fluorescence spectra of polymers are comparably broad and seem to be similar and less characteristic [11] [12] . However, we found that the spectra can be precisely characterized by means of Gaussian functions [14] according to Equation (1) where I(λ) is the fluorescence intensity dependent on the wavelength λ.
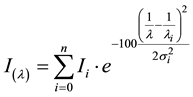 (1)
(1)
Ii is the intensity of the entire Gaussian band i, λi the position and σi the widths. λ is in the denominator because of the reciprocal relation with the energy of the basic Gaussian band. The factor 100 in the exponent simplifies the interconversion between the wavelength in nm and the energy-linear kK (10,000 cm−1).
We applied Gaussian analyse to the technical high-performance polymers Luran® (ASA), Ultramid® (PA) and Delrin® (POM) as typical examples and found that n = 4 to 5 Gaussian functions are sufficient for a precise description of the experimental spectra in the visible; see Table 2.
The Fluorescence spectrum of Luran® in the visible (>400 nm) is perfectly described by means of four Gaussian bands; see Figure 5. Thus, 12 parameters (see Table 2) are sufficient for the complete description of the broad spectrum and require minimal 12 values [15] of fluorescence intensity at different wavelengths.
Similar results are obtained for Ultramid® where also four Gaussian bands are sufficient for characterization; see Figure 6.
The behaviour of Delrin® is slightly different because of strong fluorescence in the UV close to the visible where five bands concern the visible and one or two bands in the UV should be considered because of tailing into the visible; see Figure 7.
![]()
Figure 5. Fluorescence spectrum of Luran® (solid, thick curve) and simulated spectrum (thin, dashed curve, mainly covered by the experimental spectrum) composed of the bands of a Gaussian analysis (>400 nm) on the basis of the parameters of Table 2. Bars: Positions and intensities of the Gaussian bands.
![]()
Table 2. Parameter of the sum of Gaussian functions according to Equation (1) for the technical polymers Ultramid® (PA), Luran® (ASA) und Delrin® (POM). The numbers i of the bands are given in brackets, the wavelengths λ in nm and σ in kK (10,000 cm−1). The intensities I are derived from the normalized experimental spectrum in the investigated interval.
![]()
Figure 6. Fluorescence spectrum of Ultramid® (solid, thick curve) and simulated spectrum (thin, dashed curve, mainly covered by the experimental spectrum) composed of the bands of a Gaussian analysis (>400 nm) on the basis of the parameters of Table 2. Bars: Positions and intensities of the Gaussian bands.
![]()
Figure 7. Fluorescence spectrum of Delrin® (solid, thick curve) and simulated spectrum (thin, dashed curve, mainly covered by the experimental spectrum) composed of the bands of a Gaussian analysis (>375 nm) on the basis of the parameters of Table 2. Bars: Positions and intensities of the Gaussian bands.
3.5. Practical Setup
For the determination of the first order time constant it is not necessary to record the whole exponential decay but the sampling of two fluorescence intensities [16] - [18] , conveniently before and after the first half life is sufficient; for further details, see ref. [13] . This sampling needs not to be at definite times, but may be obtained by integration over a defined interval and thus, increases the signal to noise ratio. Moreover, an absolute determination of intensities is not necessary because of unproblematic calibration. Four points are necessary for bi-expo- nential decays and for some cases three would be enough.
As a consequence, a convenient setup is the application of a laser with ns or sub ns light pulses for optical excitation and phase shifted detections of the fluorescence intensities; two detections are necessary for mono-ex- ponential decay and four for bi-exponential. A spectral resolution to 12 until 15 wavelengths allows the further identification by means of the data of the Gaussian analyses. The optical detection can be accumulated for the improvement of the signal to noise ratio where a frequency of repetition of 15 MHz may be conveniently reached. Thus, half a ton of material can be separated per hour by the application of convenient techniques.
4. Conclusion
Technical polymers can be identified by means of the mono-exponential lifetime of their autofluorescence. A bi- exponential recording allows an advanced classification useful for the discrimination of chemical similar materials such as LDPE, HDPE and UHDPE, different processing or the recognition of contaminates. The fluorescence spectra of polymers can be simplified by means of Gaussian analyses and applied as a source for redundant information of fine tuning for the recognition of certain batches. The optical method allows automation for sorting plastics in the dimensions of half a ton per hour.
Acknowledgements
This work was supported by The BMBF, CIPSM cluster in Munich, and the Fonds der Chemischen Industrie. We thank Dr. Moritz Ehrl for technical assistance. We thank Karin Haslböck and Dipl.-Ing. Dipl.-Wl-Ing. Thomas Böhme for material support.
NOTES
*Corresponding author.