Preparation, Characterization and Statistical Studies of the Physicochemical Results of Series of “B” Carbonated Calcium Hydroxyapatites Containing Mg2+ and CO2-3 ()
1. Introduction
A number of studies have reported that the incorporation of magnesium in hydroxyapatites Ca10(PO4)6(OH)2 is limited [1] - [3] . Previously, it has been shown that the magnesium can disturb the crystallization of apatites when its concentration in the solution is sufficient to be a major competitor for calcium [4] . But when the molar ratio of Mg/Ca is higher than 0.1, another phase is observed, the whitlockite [3] [5] - [7] . The co-substitution of a second ionic species like the carbonate ion can increase the insertion of magnesium in the lattice and prevent the decomposition while stabilizing the structure [8] [9] .
On the other hand, it is now well established that the biological minerals are best described as carbonated apatites rather than as a hydroxyapatite [2] [10] - [12] . The carbonate presents at 3% - 6% in biological apatites, mostly substitutes for the phosphate ion in the crystal structure and has a significant influence on the incorporation of other foreign ions into the apatite lattice. Magnesium is one of the most abundant trace ions present in the biological hard tissues and in dental enamel, its content approximately being 0.1% - 0.4%. In dentin, the magnesium content is up to 1.1%, while in bone, it is found at 0.6% [13] - [15] . Thusly, Magnesium has been the subject of many studies. To understand the role of magnesium on biological apatites, the works using synthetic carbonated apatites are very helpful.
Previous studies suggest that the magnesium is incorporated into or onto the carbonated apatites during their formation [16] - [24] . Some of these works demonstrate the role of the carbonate concentration, the pH of preparation, and the magnesium content incorporated into the apatites at similar quantities to those found in biological apatites [16] [17] [23] . Other works report the effect of the magnesium on the parameters of the lattice of apatites prepared by precipitation or high-temperature synthesis [4] [25] . Legeros et al. [22] noted an increase in the dissolution rates, of carbonate-containing apatites when the magnesium was incorporated. Some studies have investigated the phase’s composition after heat-treatment of the magnesium/carbonate co-substituted in the hydroxyapatite [16] [23] [24] .
Despite numerous investigations, the mechanism(s) by which the carbonate and the magnesium are incorporated in the apatite lattice are not yet known. Indications are found in the literature about the mechanisms by
which 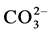 and alkalimetal M+ are incorporated in the apatite lattice [26] - [28] . In these works, De Maeyer
and alkalimetal M+ are incorporated in the apatite lattice [26] - [28] . In these works, De Maeyer
and Verbeeck suggest that six fundamental substitution mechanisms can contribute theoretically for these substitutions .
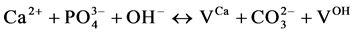 (I)
(I)
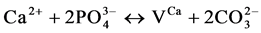 (II)
(II)
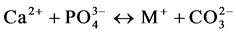 (III)
(III)
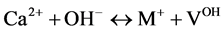 (IV)
(IV)
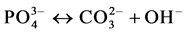 (V)
(V)
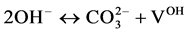 (VI)
(VI)
where VX stands for a vacancy in the X-sublattice. The contributions of each of these mechanisms should be estimated on the basis of a thorough physicochemical studies of the samples.
The present study tries to find the mechanism(s) which contribute to the incorporation of magnesium and carbonate in the apatites lattice. For this purpose, series of “B” carbonated calcium hydroxyapatites containing
magnesium are prepared by the precipitation method. In the first series, the concentration of the 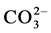 solution
solution
is Cc = 0.00 M while the Mg2+ concentration CMg is 0.00, 1.7, 6.8 and 13.6 mM. For the second, the same procedure is remade with Cc = 0.025 M in the hydrolysis solution and for the third, Cc is equal to 0.05 M. The chemical and physical characteristics of the samples prepared are determined and an attempt is made to deduce the fundamental substitution mechanisms which determine their stoichiometry. Finally, statistical studies of the experiment results allow us to find the relationship between the different variables and to verify the proposed mechanisms by which ![]() and Mg2+ are incorporated in the apatite lattice.
and Mg2+ are incorporated in the apatite lattice.
2. Methods and Materials
2.1. Preparation of “B” Type Carbonated Hydroxyapatites Containing Magnesium
The method of preparation used in this work is inspired from the method used in reference [23] but it is slightly modified. The apaties are prepared by dropping 200 mL of a phosphate solution (NH4)2HPO4 (0.18 M) into 200 mL of a calcium solution Ca(NO3)·4H2O (0.44 M) under reflux at 87˚C. To the calcium solution is added 20 mL of a magnesium solution Mg(NO3)2·6H2O containing different concentrations: CMg (0.00; 1.7; 6.8 and 13.6) mM. The same procedure is remade by adding to the phosphate solution 5 mL of a carbonate solution NH4HCO3 (1 M). A third set of preparations is performed by adding 10 mL from the above carbonate solution. The pH is maintained at 9.0 during the precipitation by adding an ammonia concentrated solution (28% weight). The precipitation is carried out over 3 h. Then, the system is refluxed for an additional duration of 2 h. The samples are filtered, thoroughly washed with hot distilled water and dried overnight at 120˚C.
2.2. Physical Analysis
The powdered samples are identified by X-ray diffraction and by infra red spectroscopy. Infrared spectra of the samples dispersed in KBr tablets are recorded using a Shimadzu Fourier transform infrared spectrophotometer in the range of 4000 - 400 cm−1. Then, the samples are analyzed by X-ray diffraction (XRD) using a Philips diffractometer using Cu Ka radiation. The samples are scanned in the 2θ range of 20˚ - 60˚. The “a and c” parameters of the lattice of the hexagonal unit cell are calculated using “wincell” refinement program.
2.3. Chemical Analysis
The samples are analyzed for Ca, PO4, CO3 and Mg. The calcium content of the precipitates is determined by a complexometric titration with the ethylenediaminetetraacetic acid [29] , the magnesium by atomic absorption, the carbonate content is determined by coulometrically method and the phosphorus content by spectrophotometrie of the phosphomolybdate complex [30] .
3. Results
3.1. Results of Physical Analysis
The IR Spectra of some representative samples (Mg4, Mg8 and Mg12) are shown in Figure 1. The spectra contain the characteristic bands of the phosphate group ![]() in the ranges 960 - 1100 and 570 - 610 cm−1. Two broad bands, around 1635 cm−1 and 3400 cm−1, confirm that the samples contain a significant amount of water. On the
in the ranges 960 - 1100 and 570 - 610 cm−1. Two broad bands, around 1635 cm−1 and 3400 cm−1, confirm that the samples contain a significant amount of water. On the
![]()
Figure 1. IR spectra of some representative samples.
spectra of the samples (Mg8 and Mg12) are displayed typical absorption bands of ![]() at ~873 and ~1420 cm−1 and between 1450 and 1500 cm−1, characterizing the vibration of the
at ~873 and ~1420 cm−1 and between 1450 and 1500 cm−1, characterizing the vibration of the ![]() on
on ![]() lattice sites (B- type
lattice sites (B- type![]() ) [31] . From Figure 1, we can clearly see that the intensity of these absorptions increases with the increase of the carbonate content. On the other hand, the IR spectra of the compounds (Mg4, Mg8 and Mg12) show that the magnesium incorporated in the apatites causes the loss of resolution of the
) [31] . From Figure 1, we can clearly see that the intensity of these absorptions increases with the increase of the carbonate content. On the other hand, the IR spectra of the compounds (Mg4, Mg8 and Mg12) show that the magnesium incorporated in the apatites causes the loss of resolution of the ![]() absorptions bands suggesting a decrease in the crystallinity [31] .
absorptions bands suggesting a decrease in the crystallinity [31] .
The X-ray diffraction patterns of some representative samples are shown in Figure 2. The X-ray diffraction powder patterns of the compounds show only one crystal phase. The peaks are sharp, well resolved and characteristic of the hexagonal apatite phase. No extraneous peaks attributable to other phases than apatite could be found in the diffractograms. The increase of the level of ![]() substitution produces a loss of the resolution of the 112 peak and a decrease in the intensity of the 300, 202 and 002 peaks.
substitution produces a loss of the resolution of the 112 peak and a decrease in the intensity of the 300, 202 and 002 peaks.
The Table 1 contains the values of the lattice parameters “a” and “c” obtained for the different compounds.
From this table, we can see that simultaneous incorporation of two elements “CO3 and Mg” results in an decrease of the “a” parameter. This contraction is attributed to the simultaneous effects of the ![]() and Mg2+ substitutions [2] .
and Mg2+ substitutions [2] .
![]()
Figure 2. X-ray diffraction patterns of some representative samples.
![]()
Table 1. “a” and “c” Lattice parameters of “B” CO3Mg-Haps.
3.2. Chemical Results
The results of the chemical analysis of the samples in Weight % are summarized in Table 2. This table also gives the hydroxide content of the samples calculated on the basis of the electroneutrality condition and the total mass balance ∑ % obtained from the equation:
![]() (1)
(1)
With MX the atomic or ionic mass of X. ∑ % value is lower than 100% indicating that the samples of the present study still contain some water after drying at 120˚C.
The results of the chemical and physical analysis (Table 2) allow us to calculate the number of each ion X per unit cell, nx according to the following equation:
![]() (2)
(2)
The results of these calculations are summarized in Table 3. The errors in Table 4 are estimated by the means of error propagation theory.
![]()
Table 2. Chemical Composition (weight percent) and Total Mass Balance ∑ % of the hydroxyapatites obtained by precipitation in solutions containing Cc (M) CO3 and CMg (mM) Mg.
![]()
Table 3. Unit cell compositions of NaCO3 Aaps calculated on the basis of the chemical composition and using Equation (2).
4. Statistical Analysis of the Physicochemical Results
4.1. Influence of the Experimental Conditions on the Composition of the Synthetic Apatites
To know the influence of the experimental conditions on the incorporation of ![]() and Mg2+ in the lattice of these synthetic apatites, we graph Yi =
and Mg2+ in the lattice of these synthetic apatites, we graph Yi = ![]() /nP and nMg/nP the molar ratios contents of the samples against Xi the concentration of
/nP and nMg/nP the molar ratios contents of the samples against Xi the concentration of ![]() Cc or the concentration of Mg2+ CMg in the solution, Figure 3 and Figure 4.
Cc or the concentration of Mg2+ CMg in the solution, Figure 3 and Figure 4.
From the Figure 3(a) and Figure 4(a), it is seen that ![]() /nP the molar ratio increases with the increase of the concentration of
/nP the molar ratio increases with the increase of the concentration of ![]() in the solution (Cc/M). Contrariwise, it varies slightly with the concentration of the Mg2+ ions in the solution and vice versa for nMg/nP (Figure 3(b) and Figure 4(b)).
in the solution (Cc/M). Contrariwise, it varies slightly with the concentration of the Mg2+ ions in the solution and vice versa for nMg/nP (Figure 3(b) and Figure 4(b)).
To estimate the simultaneous influence of the experimental conditions on ![]() /nP and nMg/nP the molar ratios, we construct a mathematical model of Yi =
/nP and nMg/nP the molar ratios, we construct a mathematical model of Yi = ![]() /nP or nMg/nP on two variables X1,i = Cc and X2,i = CMg.
/nP or nMg/nP on two variables X1,i = Cc and X2,i = CMg.
The mathematical model is described by the equation:
![]() (3)
(3)
The method of least squares (O.L.S.) allows us to establish the predicted equation
![]() (4)
(4)
that is most suitable to the data. On the other hand, this method allows us to calculate the estimated standard errors of the coefficients![]() , the individual confidence interval at 95% level, R2 the standardized statistic and to test the null hypothesis H0: bj = 0 and its significances level. The analysis of the variance for the linear regression or the F test allows us to ensure that at least one of the X-variables contributes to the regression. The theoretical basis of these calculations is given in references [32] -[34] . The calculations are summarized in Table 4 and Table 5.
, the individual confidence interval at 95% level, R2 the standardized statistic and to test the null hypothesis H0: bj = 0 and its significances level. The analysis of the variance for the linear regression or the F test allows us to ensure that at least one of the X-variables contributes to the regression. The theoretical basis of these calculations is given in references [32] -[34] . The calculations are summarized in Table 4 and Table 5.
4.2. Influence of the Incorporation of ![]() and Mg2+ on the Variation of Ca2+ and OH− the Molar Ions Contents of the Synthetic Apatites
and Mg2+ on the Variation of Ca2+ and OH− the Molar Ions Contents of the Synthetic Apatites
In attempts to disentangle and to measure the effects of the insertion of ![]() and Mg2+ ions on the molar con-
and Mg2+ ions on the molar con-
![]()
![]() (a) (b)
(a) (b)
Figure 3. (a) ![]() /nP molar ratio of the solid versus CMg/mM for the samples prepared at different Cc/M; (b) nMg/nP molar ratio of the solid versus CMg/mM for the samples prepared at different Cc/M.
/nP molar ratio of the solid versus CMg/mM for the samples prepared at different Cc/M; (b) nMg/nP molar ratio of the solid versus CMg/mM for the samples prepared at different Cc/M.
![]()
![]() (a) (b)
(a) (b)
Figure 4. (a) ![]() /nP molar ratio versus Cc/M for the samples prepared at different CMg/mM; (b) nMg/nP molar ratio versus Cc/M for the samples prepared at different CMg/mM.
/nP molar ratio versus Cc/M for the samples prepared at different CMg/mM; (b) nMg/nP molar ratio versus Cc/M for the samples prepared at different CMg/mM.
(a) ![]() (b)
(b) ![]() (c)
(c) ![]()
Table 5. Multiple linear regression analysis of Yi = nMg/nP molar ratio (Table 3) as a function of the concentration of carbonate Cc/M and magnesium CMg/mM in the solution. (a) Regression statistic; (b) Coefficients; (c) Analysis of variance.
tent of Ca2+ of the solid, we use the multiple linear regression on two X-variables where, X1 = ![]() and X2 = nMg2+ and Y is the estimate molar content of Ca2+ or OH− (data Table 3). The results of these calculations are given in Table 6 and Table 7.
and X2 = nMg2+ and Y is the estimate molar content of Ca2+ or OH− (data Table 3). The results of these calculations are given in Table 6 and Table 7.
4.3. The Determination of the Relationship between Y = c/a Crystallographic Parameters Ratio and ![]() /
/![]() the Molar Ratio
the Molar Ratio
To estimate the influence of the incorporation of carbonate on the lattice parameters “a” and “c” in presence of magnesium, we plot c/a crystallographic parameters ratio (Table 1) as a function of molar ratio ![]() /nP (Table 3) for 0 ≤ nMg ≤ 17.4 mM (Figure 5).
/nP (Table 3) for 0 ≤ nMg ≤ 17.4 mM (Figure 5).
Given that the shape of the curve obtained in Figure 5 is a polynomial, we construct a multiple linear regression on Yi = c/a as a function of three X-variables where, X1 = ![]() /nP, X2 = (
/nP, X2 = (![]() /nP)2 and X3 = (
/nP)2 and X3 = (![]() /nP)3 (data Table 2) and Y is the estimate ratio of the hexagonal lattice dimensions (data Table 1). The mathematical model equation is
/nP)3 (data Table 2) and Y is the estimate ratio of the hexagonal lattice dimensions (data Table 1). The mathematical model equation is
![]() (5)
(5)
Least square [33] allows calculating the regression and correlation coefficients regression of the predicted
equation
![]() (6)
(6)
These estimated rgression coefficients ![]() are calculated from the values of correlation coeffici- ents, variance and covariance according to the method of Scherrer [33] . This method allows us to test the utility of the model or the F-test according to:
are calculated from the values of correlation coeffici- ents, variance and covariance according to the method of Scherrer [33] . This method allows us to test the utility of the model or the F-test according to:
![]() (7)
(7)
where n is sample size, m is number of parameters and (n − m − 1) is degree of freedom.
On the other hand, this method allows us to calculate the standard errors of the coefficients ![]() and to conduct t-tests on the b’s (to discover which variable(s) is related to estimate
and to conduct t-tests on the b’s (to discover which variable(s) is related to estimate![]() ) and to calculate the individual confidence interval at 95% level. The results of these calculations are given in Table 8.
) and to calculate the individual confidence interval at 95% level. The results of these calculations are given in Table 8.
The analysis of variance (ANOVA) shows that F-test = 2018.9 is higher than criterion F(5%; 3; 8) = 4.07.
![]()
Figure 5. c/a parameters ratio as a function of ![]() /nP the molar ratio for the apatites prepared at different values of CMg.
/nP the molar ratio for the apatites prepared at different values of CMg.
5. Determination of the General Formula of the Unit Cell of the Synthetic “B” CO3Mg-HAps
The relative composition (Table 3) and the results of the physical analysis demonstrate that the samples are pure “B” type carbonated apatites containing Mg2+ ions. Thus, mechanisms I, II, III and V could be account in the incorporation of ![]() on
on ![]() and Mg2+ ions are incorporated in the apatite lattice according to mechanisms III and/ or IV.
and Mg2+ ions are incorporated in the apatite lattice according to mechanisms III and/ or IV.
Moreover, the study carried out previously (paragraph 4.1) show that the ![]() ions are incorporated in the apatite lattice independently of the concentration of Mg2+ ions solution. This result confirms that mechanism IV does and mechanism III does not contribute to the incorporation of Mg2+ in the apatites.
ions are incorporated in the apatite lattice independently of the concentration of Mg2+ ions solution. This result confirms that mechanism IV does and mechanism III does not contribute to the incorporation of Mg2+ in the apatites.
Many works [27] [28] have demonstrated that mechanism I and/or II are the main mechanisms for the incorporation of CO3. Otherwise, according the reference [27] , the contribution of mechanism I seems to be hardly influenced by the alkali metal which is not our case. Therefore, we consider that mechanism II contribute to the insertion of CO3 ions in the lattice of the solid.
Table 7 show that the variation of nOH− depends on the increase of ![]() and Mg2+. So, it may be said in the present study, that the mechanism V could account.
and Mg2+. So, it may be said in the present study, that the mechanism V could account.
Then the fundamental substitution mechanisms for the incorporation of ![]() and Mg2+ in the HAp lattice are:
and Mg2+ in the HAp lattice are:
![]() (II)
(II)
![]() 2.(IV)
2.(IV)
![]() (V)
(V)
where VOH stands for a vacancy in the OH− sub lattice. If x, y and z are the contributions of mechanisms II, 2.IV and V respectively, thus,
![]() (8)
(8)
![]() (9)
(9)
![]() (10)
(10)
![]() (11)
(11)
and ![]() (12)
(12)
and the generic formula has the following expression:
![]()
The values of x, y and z the contribution of mechanisms II, 2.IV and V respectively are calculated from the data (Table 3) and the following equations. Then statistical studies are conducted to verify the accuracy of the proposed formula. The results of these calculations are summarized in Tables 9-11.
![]()
Table 9. The values of x, y and z the contributions of the mechanisms II, 2.IV and V respectively calculated from Equations (10)-(14).
(a) ![]() (b)
(b) ![]() (c)
(c) ![]()
Table 10. Multiple linear regression analysis of the estimated Yi = nCa2+ the molar content of the solid “B” Mg-CO3 HAps (Table 3) on X1,i = x- and X2,i = y the contribution of mechanisms II and 2.IV (Table 9). (a) Regression statistic; (b) Coefficients; (c) Analysis of variance.
(a) ![]() (b)
(b) ![]() (c)
(c) ![]()
Table 11. Multiple linear regression analysis of the estimated Yi = nOH− the molar content of the solid “B” Mg-CO3 HAps (Table 3) on X1,i = z and X2,i = z the contribution of mechanisms II and V (Table 9). (a) Regression statistic; (b) Coefficients; (c) Analysis of variance.
6. Discussion
From Table 1,, we can see that simultaneous incorporation of two elements “CO3 and Mg” results in an decrease of the “a” parameter. This contraction is attributed to the simultaneous effects of the ![]() and Mg2+ substitutions [4] .
and Mg2+ substitutions [4] .
In Table 2 and Table 3, it is seen that the concentration of the ![]() ions in the solution Cc does not affect the quantities of Mg2+ ions inserted in the solid. Because, regardless ofthe concentration of the
ions in the solution Cc does not affect the quantities of Mg2+ ions inserted in the solid. Because, regardless ofthe concentration of the ![]() ions in the solution Cc, the Mg2+ ions contents of the samples increase proportionally with the increase of the concentration of Mg2+ ions in solution CMg. This result is in agreement with reference [4] . For the same concentration of Mg2+ions in the solution CMg, the variation of
ions in the solution Cc, the Mg2+ ions contents of the samples increase proportionally with the increase of the concentration of Mg2+ ions in solution CMg. This result is in agreement with reference [4] . For the same concentration of Mg2+ions in the solution CMg, the variation of ![]() and
and ![]() contents of the solid do not seem to be correlated with the concentration of the Mg2+ ions in the solution, while the Ca2+content depends on the concentrations of
contents of the solid do not seem to be correlated with the concentration of the Mg2+ ions in the solution, while the Ca2+content depends on the concentrations of ![]() Cc and Mg2+ CMg in the solution.
Cc and Mg2+ CMg in the solution.
Figure 3 and Figure 4, show that nCO3/nP the molar ratio increases with the increasing of the concentration of ![]() in solution (Cc/M). Contrariwise, it varies slightly with the concentration of Mg2+ ions in solution and vice versa for nMg/nP. The statistical treatment of the experimental data Table 4 and Table 5 allows us to establish the estimated equations between these variables at 95% levels
in solution (Cc/M). Contrariwise, it varies slightly with the concentration of Mg2+ ions in solution and vice versa for nMg/nP. The statistical treatment of the experimental data Table 4 and Table 5 allows us to establish the estimated equations between these variables at 95% levels
![]() (13)
(13)
and ![]() (14)
(14)
Equations (9) and (10) show that the concentration of the ![]() ions in the hydrolysis solution Cc affects the quantities of
ions in the hydrolysis solution Cc affects the quantities of ![]() and Mg2+ ions incorporated in the solid, but the concentration of Mg2+ ions in solution CMg does not affects the quantities of
and Mg2+ ions incorporated in the solid, but the concentration of Mg2+ ions in solution CMg does not affects the quantities of ![]() ions in the solid. These results are in agreement with those found in the reference [27] .
ions in the solid. These results are in agreement with those found in the reference [27] .
To know the relationship between the variation of Ca2+ and OH− with the increasing of ![]() and Mg2+ content in the solid, statistical studies are conducted. The results of multiple linear regression Table 6 and Table 7 show that the estimated equations on these variables are represented at 95% level by:
and Mg2+ content in the solid, statistical studies are conducted. The results of multiple linear regression Table 6 and Table 7 show that the estimated equations on these variables are represented at 95% level by:
![]() (15)
(15)
![]() (16)
(16)
From the intercepts of the following equations it can seen that, within experimental error, a carbonate and magnesium-free apatite (nCO3 = 0, nMg = 0) contains 10 Ca2+ and 2 OH− ions per unit cell Equations (11) and (12). These results are in agreement with those in literature [24] - [26] [34] [35] .
As shown in Figure 5, there is a correlation of the unit cell parameters of the apatites with their chemical compositions. Indeed, the changes in the unit cell parameter “a” of the compounds are attributed to the additive effects of the substitution in the lattice of either carbonate and magnesium [2] [34] [35] . The solid line of best fit for these series of compounds in Figure 5 extrapolates to a ratio c/a very close to that in hydroxyapatite. This result is similary to these obtained previously [34] [35] . The application of multiple linear regression to Yi = c/a
on ![]()
![]() allows us to establish the predicted equation at
allows us to establish the predicted equation at
95% level:
![]() (17)
(17)
To verify the general formula proposed, We apply the multiple linear regression to Yi = nCa2+ on X1i = x and X2i = y (the contributions of the mechanisms II and IV). Similar treatment is realized for Yi = nOH− on X1i = z and X2i = y (the contributions of the mechanisms V and IV) Table 10 and Table 11. The results of these calculations show that the predicted equations at 95% level are:
![]() (18)
(18)
![]() (19)
(19)
7. Conclusion
The theoretical calculations of the present study indicate unambiguously that the mechanisms II, III and V contribute to the incorporation of Mg and Ca in the lattice of apatite. This corroborates in more definite way our assumptions obtained from the experimental data and allows us to propose for these compounds the general formula:
![]()