Effects of Ion Doping on the Optical Properties of Dye-Sensitized Solar Cells ()
1. Introduction
Due to the low price, easy fabrication process, high conversion efficiency and good stability, since the DSC in 1991, [1] has made breakthrough progress, and has aroused widespread concern in the academic circles and the business community [2] - [6] . In recent years, doping TiO2 with metal and nonmental elements has been considered as a promising way to tailor the electronic properties of TiO2 photoanode in DSC and has succeeded in improving photovoltaic performance of DSC [7] - [11] . Performance of doped metal ions on monocrystalline or polycrystalline TiO2 present in the crystal lattice has become good electron trap. It can reduce electron-hole pair recombination, extend the life of charge, thus to improve the efficiency of DSC [12] . This paper studied the influence of different ion doping and different concentration of ion doping on the electrical and optical properties of DSC, and confirmed the best concentration and the best types of ion doping through optical performance testing.
2. Experimental
2.1. The Film Preparation Method
The sol-gel method for preparing anode TiO2 films, sol-gel method is a common method for preparing wet chemical materials [1] [13] - [16] . Sol-gel derived samples high uniformity, high purity of products, easy control of the reaction process, has great advantages in the application of the film, becoming one of the most commonly used method for preparing thin films. TiO2 film composition prepared using different process methods or parameters, structure, orientation and thickness are the differences.
2.2. Preparation of TiO2 Sol-Gel
Tetrabutyl titanate hydrolysis reaction in an acidic solution [17] :
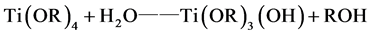 (1)
(1)
 (2)
(2)
 (3)
(3)
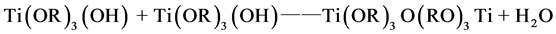 (4)
(4)
The hydrolyzed reaction mainly (1) and (2) out of the way, the product was incomplete hydrolyzate Ti(OR)3(OH) and Ti(OH)4, Ti(OH)4 lose a water molecules generated TiO2 colloidal particles. Polycondensation reaction (3) and (4) happened after 10 hours, polycondensation product gel form titanium oxide.
Chemically pure tetrabutyl titanate as raw material in this experiment, using ethanol as solvent, diethanolamine as complexing agent, nitric acid as catalyst. Experiment steps were as follows:
1) Prepare a mixture A with tetrabutyl titanate, ethanol and diethanolamine, fully stirred to give a homogeneous mixture.
2) Prepare a uniformly mixed mixture A with ethanol, deionized water and nitric acid.
3) Under magnetic stirring, the above mixture B was added into the mixture A dropwise to obtain a uniform, light yellow transparent sol, and the hydrolysis polycondensation reaction at room temperature to obtain sol C.
The mole ratio of tetrabutyl titanate, ethanol, diethanolamine and and nitric acid was 12:48:4:1:0.6.
2.3. Ion Doping
The desired ionic salt was put into B solution. Adding the solution B in solution A dropwise under magnetic stirring to give a homogeneous, light yellow transparent sol. The hydrolysis polycondensation reaction at room temperature to obtain solution C.
2.4. Cell Production
The TiO2 film was prepared by sol-gel method, drying at room temperature and then placing the crucible in a muffle furnace at 20˚C/min at 500˚C heated to a constant temperature for two hours, then cooled in the furnace. After hydrolysis of the intermediate product suitable high-temperature annealing was completely decomposed, residual organic matter can be completely removed, and finally completely dehydrated, only closely integrated with the substrate of titanium dioxide films.
Using DMF solution with Eosin Y as sensitizer, the concentration of 0.001 g/mL. Putting the annealed TiO2 film into the dye solution and rushing floated color with ethanol after 24 hours to make the anode. Using acetonitrile solution with KI and I2 as electrolyte. The counter electrode was prepared by electroplating method on FTO conductive glass with pt.
The prepared TiO2 film colored face up on the table, the pore size of 0.5 cm2 of silicone sheet on the anode and the counter electrode on top, with a clip to clip up on both sides of the battery, electrolyte injected with a syringe. Cell production is complete to measure optical properties.
3. Results and Discussion
3.1. Effect of Different Ion-Doped Optical Performance of DSC
Testing the DSC with Beijing changtuo company CHF-XM-500W xenon lamp as the light source, the incident light intensity was 73.1 mW/cm2, the open circuit voltage of Voc and short circuit current density Isc. The open circuit voltage can be seen from Figure 1, Nd3+ doped TiO2 thin films was lower, Zn2+ doped TiO2 thin film photovoltaic best performance, cell power was highest, indicating that the effect of doping of Zn2+ was the best in these three kinds of ions.
As can be seen from the Figure 1, not only the curve hardness of ion-doped TiO2 film was significantly better than undoped TiO2 film, but also the current and voltage was higher than the undoped film. It illustrated the truth of certain mount of doping ions can improve the property of TiO2 thin films. This was because the oxide melting point have a certain influence on the phase transformation of TiO2, when the oxide melting point was higher than TiO2, can inhibit the transformation of anatase to rutile, and when the oxide melting point lower than TiO2, can promote the transformation, and the lower the melting point effect more obvious.
Using the RIGAKU D/MAX-2200 type PC X-ray diffraction of the phase of the sample were measured for the Cu target radiation. λ = 0.15418 nm, the working voltage of 40 kV, current of 30 mA, the scanning range of 10˚ - 80˚, the scanning speed was 20/min. As can be seen from the Figure 2, doping ions easily lead redox reaction in the titania lattice surface, then produced oxygen vacancy or interstitial titanium by diffusion, thereby inhibiting the interaction between different titanium atoms, transition hinder anatase to rutile phase, to improve the light absorption ability of TiO2 thin films.
![]() (a)
(a)![]()
![]() (b) (c)
(b) (c)
Figure 1. The I - V curve of ion doped DSC. (a) The I - V curve of Zn2+ doped DSC; (b) The I - V curve of La3+ doped DSC; (c) The I - V curve of Nd3+ doped DSC.
![]()
Figure 2. XRD patterns of Titania films.
3.2. UV-Vis Characterization of Different ion Doped TiO2 Thin Film
The characteristics of samples were examined through a UV-Vis spectrophotometer type UV-2550 produced by Japanese Shimadzu Company, scan speed was medium, the slit width was 2 nm, wavelength range was 200 nm to 800 nm.
Figure 3 was the UV-Vis spectra of TiO2 films doped with ions in the 300 - 600 nm. It can be seen from that the spectra in the wavelength range of 500 nm or more, whether the TiO2 film was doped with ions or not, light absorption was relatively small. Absorb light beginning between 300 - 400 nm, and the wavelength was shifted to shorter wavelength direction as the absorption peak was more obvious. While in the UV range, TiO2 films have a strong absorption of light.
The spectral curve changed on the form compared with the non-doped TiO2 films. The absorption peak shifted to longer wavelengths, spectral red shift. Expand the scope of TiO2 nanoparticles in response to visible light direction, improved the absorption properties in a certain extent. The band gap of TiO2 thin film doped with Zn2+ is 2.77 eV, the band gap of TiO2 thin film doped with La3+ is 2.51 eV, the band gap of TiO2 thin film doped with Nd3+ is 2.71 eV, all smaller than the band gap of undoped TiO2 films (3.0 eV), demonstrated that the doping ion can improve the photocatalytic activity of TiO2 thin films.
3.3. Effects of Doping Concentration on the Photoelectric Properties of DSC
Many studies have shown that ion doping with an optimal concentration. With increasing the concentration of the doping, the surface space charge layer is narrowed, electrons and holes are generated by light excitation TiO2 and can be effectively separated, the lifetime of photo-induced carriers prolonged, but when the doping concentration is lower than the optimal concentration, there is not enough traps in the semiconductor to capture the photo-induced carriers, electrons and holes can not reach the most effective separation; When the optimum doping concentration, the space charge layer thickness is exactly equal to the incident light penetration depth, the photo-generated electrons and holes have the optimal separation, the most favorable to the photocatalytic reaction; It would cause an increase of photo-induced carriers recombination in the surface when excess dopant to reduce the photocatalytic efficiency.
The doping of Zn2+ as an example to illustrate the effects of different doping concentration on the properties of DSC. The doping concentration in solution was respectively 0.001%, 0.03%, 0.05%, 0.1%, 0.2% (mole fraction). As can be seen from Figure 4, the open circuit voltage and short circuit current increased with the ions concentration increased. However, when reached a certain value, the open circuit voltage and short circuit current decreased, which also proved the existence of an optimum value of ions concentrationt, and the optimum value was 0.05%.
![]() (a)
(a)![]()
![]() (b) (c)
(b) (c)
Figure 3. UV-Vis spectra curve of ions doped TiO2 thin films. (a) UV-Vis spectra curve of Zn2+ doped TiO2 thin film; (b) UV-Vis spectra curve of La3+ doped TiO2 thin film; (c) UV-Vis spectra curve of Nd3+ doped TiO2 thin film.
![]()
Figure 4. The I-V curve of different concentrations ions doped DSC.
Semiconductor ZnO and TiO2 belong to different band gap, in both the interface the Ti4+ into the ZnO lattice instead of Zn2+ causing a charge imbalance due to Zn2+ ionradius is larger than the radius Ti4+, in order to bal- ance the charge, more −OH was absorbed to the surface, and the surface adhesion of the −OH to accept the photogenerated holes. This reduces the probability of the photogenerated hole and electron recombination, improves the separation of photogenerated electrons and holes, which exhibits better photocatalytic activity. However, when doped Zn2+ too much, ZnO and TiO2 solid solution has reached saturation, the excess of Zn2+ in the form of ZnO deposited on the surface of TiO2 grains, reducing the effective Specific surface area of TiO2 films. And because the band gap of ZnO is greater than TiO2, the UV-visible irradiation does not cause photocatalytic reaction, so the accumulation of ZnO on the surface of TiO2 hinder the photocatalytic reaction, causing lower photocatalytic activity of TiO2 films.
The Zn2+ optimum doping amount is 0.05%, because then the transfer rate of carriers of the fastest, the separation of electron hole pair efficiency is the highest, the photocatalytic activity is the strongest. When Zn2+ doping amount is less than 0.05%, the defect levels and impurity levels is separating center of electron-hole pairs; When Zn2+ doping greater than 0.5%, the defect levels and impurity levels is recombination center of electron-hole pairs. carrier recombination rate accelerated, electron-hole pairs separation efficiency decreases.
3.4. UV-Vis Spectra of Different Concentration Ion Doped TiO2 Thin Film
Figure 5 was the UV-Vis spectra of TiO2 films doped with Zn2+ between 300 nm and 600 nm, including 1, 2, 3, 4, 5 respectively represent the doping concentrations were 0.001%, 0.03%, 0.05%, 0.1%, 0.2% (mole fraction). As we can be seen from the spectra, the shape of the curve changed with increasing the concentration, the absorption peak shifted to longer wavelengths. However, when the concentration continues to increase, the absorption peak moves to shorter wavelength. It also showed the presence of an optimum doping concentration, and the most preferably concentration of 0.05% (mole fraction). This was consistent with previous analyzes. It presented the first band gap decreased after the increase. It also showed that there was an optimal doping concentration. The band gap of the mixture narrowed compared with pure TiO2, the right concentration of Zn2+ doped TiO2 improved ability to absorb long-wave photons.
4. Conclusion
This paper studied the regulation of photoelectric properties though ions doped in anode of DSC. Zn2+, La3+ and Nd3+ of three kinds of ion doping were tested on the photoelectric properties of TiO2 films, and the results showed that Zn2+ doped TiO2 photoanode was the best. At the same time there was an optimum doping concentration which was 0.05% (mole fraction). The UV-Vis spectra of doped TiO2 also confirmed this conclusion.
![]()
Figure 5. UV-Vis spectra of different concentrations of ions doped TiO2 thin films.
Future research should focus on the doping mechanism, through in-depth study of the mechanism, to avoid adverse effects on the DSC doping elements brought to more effectively improve the photoelectric conversion efficiency of DSC.
NOTES
*Corresponding author.