Effective prophylaxis with rFVIIa in young haemophiliacs with inhibitors using a schedule similar to FVIII prophylaxis in non-inhibitor patients ()
1. INTRODUCTION
The benefits of early prophylaxis over on-demand therapy in severe haemophilic children that have not acquired antibodies (inhibitors) to clotting factor replacement therapy have been established [1]. Prophylaxis acts principally to minimize joint bleeds and the subsequent development of arthropathies and other life-threatening bleeding events, such as intracranial haemorrhage. Early prophylaxis is widely accepted as the preferred mode of therapy for children with severe haemophilia.
Paediatric patients with antibodies that inhibit clotting factor replacement therapy have a high risk for recurrent joint bleeds and subsequent haemophilic arthropathy [2]. Several studies on the orthopaedic status of patients with inhibitors found more severe arthropathy even in younger patients [3,4].
rFVIIa is well established as a treatment for bleeding in haemophilia patients with inhibitors and as a surgical pre-treatment to provide haemostatic cover for haemophiliac patients with inhibitors who are undergoing orthopaedic surgery [5,6]. Recent studies have developed the concept of rFVIIa-mediated prophylaxis in nonsurgical settings for haemophilia patients with inhibitors. These studies evaluated the potential benefits of bypassing agents in seconddary prophylaxis and demonstrated decreased frequency of bleeding compared to on-demand therapy [7-11].
Konkle et al. [12] reported results from the first prospective randomized clinical trial evaluating the efficacy of rFVIIa for prophylaxis in 22 frequently-bleeding haemophilia patients with inhibitors. Their results show that secondary prophylaxis with rFVIIa can reduce bleeding frequency, increase mobility, reduce pain and provide other quality-of-life benefits for these patients; however, orthopaedic joint scores did not change during their trial. Additional studies have provided further data on rFVIIa secondary prophylaxis in patients with high-responding inhibitors [9,13].
Nevertheless, secondary prophylaxis in non-inhibitor patients may not be as effective as early prophylaxis for preventing arthropathy [14]. It is of paramount importance to determine whether an early therapeutic strategy can prevent or reduce bleeding episodes, joint damage and arthropathy in young haemophiliacs during the critical period when inhibitors are present. Current case reports on early prophylactic therapy with rFVIIa are only anecdotal [11,13,15,16]. Resulting from an increased interest in bypassing prophylactic agents, a consensus article and two reviews have recently been published [17-19].
The goal of our study was to evaluate the role of early rFVIIa prophylaxis in young haemophiliacs with inhibitors from the onset of inhibitor acquisition, and to determine whether this early preventive strategy reduces bleeding episodes and prevents joint damage to the point where haemophiliacs can benefit from other therapies, such as FVIII replacement.
2. MATERIALS AND METHODS
The Working Group of Experts for the Treatment of Haemophilia Patients with Inhibitors of the Spanish Society of Thrombosis and Haemostasis (SETH) conducted a retrospective survey among Spanish haemophilia centres. All patients with inhibitors who underwent early prophylaxis with rFVIIa were included. The criteria for inclusion were those children (≤three years old) with severe haemophilia A with ≤four joint bleeds who had been treated on-demand with Factor VIII (FVIII), or were on prophylaxis and had developed inhibitors to FVIII, and had early prophylaxis with rFVIIa.
Following selection of patients, a questionnaire was sent to the Haemophilia Centres to record clinical characteristics of the patients, including bleeding episodes and prophylactic regimens used. Written informed consent was obtained from the parents of all children. Medical records of the children were utilized to complete the questionnaire.
The number and characteristics of haemorrhages and haemarthroses occurring during rFVIIa prophylaxis were recorded (the prophylaxis period). Total bleeds occurring before the start of prophylaxis were similarly recorded. The pre-prophylaxis period was divided into two parts, between the diagnosis of haemophilia and appearance of inhibitors (the initial period) and between the diagnosis and quantification of inhibitors and the start of rFVIIa prophylaxis (the inhibitor period). Other clinical and therapeutic issues, such as the prophylaxis schedule or immune tolerance induction regimen, were also recorded.
3. RESULTS
Ten severe haemophilia A patients, who were less than three years old and had developed high-responding inhibitors after treatment with recombinant FVIII (rFVIII) were included in the study. Eight of these patients had the inversion in intron 22 and one had a codon-stop mutation; the remaining patient was not characterized. Five cases had been partially reported previously [9,16], but the purpose of those reports was not coincident with our study.
3.1. Initial Period
The mean age at diagnosis of severe haemophilia A (FVIII < 1%) was 104.2 days (range: 0 - 360 days); the mean age at the first bleeding episode was 102.3 days (range: 0 - 314 days); and the mean age at the first rFVIII infusion was 199.3 days (range: 0 - 559 days). Four of the 10 patients initiated prophylaxis with rFVIII and six continued on-demand.
The number of total haemorrhagic episodes prior to inhibitor diagnosis was 45, with the mean number of bleeds per child being 4.5 (range: 2 - 14). The majority of bleeds were spontaneous cutaneous haematomas, ecchymosis related to venipuncture or intramuscular bleeding. Four boys had severe haemorrhages, two intracranial, one hemoperitoneal and one arterial pseudoneurysm. Seven episodes of haemarthrosis occurred in four patients (Table 1).

Table 1. Summary of haemorrhagic episodes in the period between diagnosis of haemophilia and appearance of inhibitor (the initial period).
3.2. Inhibitor Period
Inhibitors against FVIII developed after a mean of 16.5 days exposure (range 8 - 28 days). The prophylaxis group developed inhibitors in 12.5 days, while the ondemand group developed inhibitors in 17.5 days. Following antibody detection, a central venous catheter was implanted in six children and high-dose rFVIII, rFVIIa, activated prothrombin complex concentrates (APCCs), or rFVIIa plus high-dose rFVIII were administered prior to surgery. The mean age at inhibitor diagnosis was 15.6 months (range: 2.2 - 34.1 months). All children were high responders, with a mean maximum peak titre of 144.7 Bethesda Units (BU) mL−1 (range: 11 - 645 BU mL−1).
The mean elapsed time between inhibitor diagnosis and start of rFVIIa prophylaxis was 7.14 months (range: 0.2 - 23.19 months). The total number of haemorrhagic episodes during this period was 36 with a mean 3.6 bleeds per child, ranging from 0 to 8. Two children did not have any haemorrhage during this period. Two patients had recurrent bleeding from the catheter insertion site. Five episodes of haemarthroses occurred in two children before prophylaxis was started. Six children did not have any haemarthrosis episodes (Table 2).
In five cases, rFVIIa was used to treat the acute bleeding episodes, and in two patients additional APCC was administered. The mean dosage of rFVIIa infused was 6.66 mg (range: 1.8 - 15.6 mg) and a mean of 25,000 IU APCC was infused.
3.3. Prophylaxis Period
The mean age at the start of rFVIIa prophylaxis was 22.7 months (range: 2.5 - 34.8 months). The mean duration of prophylaxis was 10.31 months (range: 5.6 - 32.0 months). Two patients currently continue on prophylaxis. The total number of haemorrhagic episodes occurring during the prophylaxis period was 17, representing a mean of 1.7 bleeds per child (range: 0 - 4). Five episodes of joint bleeds were reported in two patients during this period; the remaining eight children had no joint bleeds (Table 3).
In six children, rFVIIa prophylaxis was coincident, at least in part, with immune tolerance induction treatment (ITT) (Table 3). The mean age at the start of ITT in this sub-group was 16.1 months (range: 2.5 - 27.0 months) and the mean age at the start of rFVIIa prophylaxis was 20.68 months (range: 2.5 - 35.2 months). The mean duration of rFVIIa prophylaxis was 7.6 months (range: 5.6 - 12.0 months). The number of bleeds during rFVIIa prophylaxis was 10, compared to 18 episodes observed in the period between inhibitor diagnosis and start of prophylaxis. Notably, no haemarthrosis occurred during rFVIIa prophylaxis. All acute bleeding was treated with extra doses of rFVIIa. Prophylaxis was suspended in five patients because of inhibitor eradication and in one patient because a second ITT regimen was started. In pa-

Table 2. Summary of the haemorrhagic episodes in the period between appearance of inhibitor and start of rFVIIa prophylaxis (the inhibitor period).
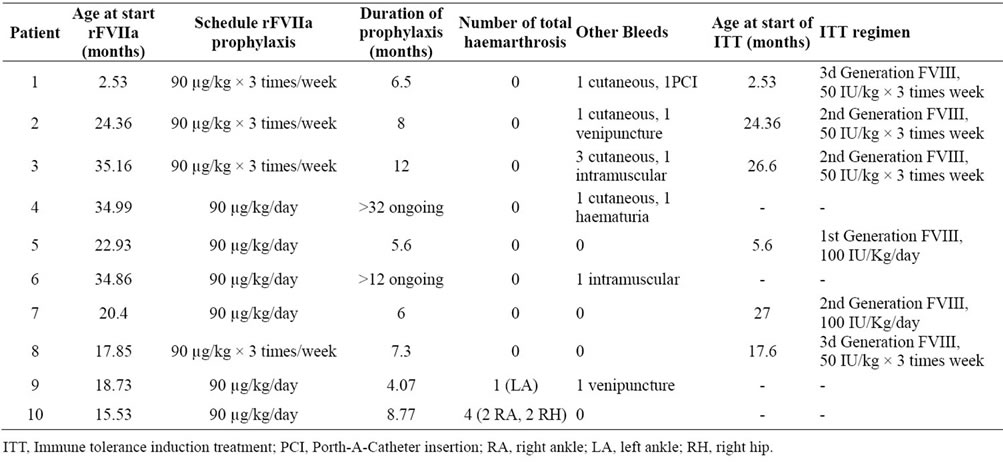
Table 3. Summary of the haemorrhagic episodes during the prophylaxis period.
tients in whom ITT was successful, regular FVIII prophylaxis was initiated.
In the other four patients, rFVIIa prophylaxis was not coincident with ITT, and was initiated to prevent bleeding before the start of ITT and continued until inhibitor titre was less than 10 BU, or because they were not expected to be included in the ITT programme. These patients were older at the start of prophylaxis with a mean age 26.0 months (range: 17.6 - 35.2 months). During rFVIIa prophylaxis, they all had fewer haemorrhagic events (9 episodes) compared with those in the previous period (18 episodes). One patient had one haemarthrosis and one patient had four (Table 3). Prophylaxis was suspended in two cases at the time of initiation of ITT, while the other two continued with the rFVIIa prophylaxis.
Comparing the three follow-up periods, bleeding episodes were 45, 36 and 17, averaging 0.29 and 0.51 haemorrhages/year/patient in the two periods prior to prophylaxis and 0.17 haemorrhages/year/patient during prophylaxis (Table 4). There were five haemarthroses in the prophylaxis period compared to the seven in the initial period and five in the inhibitor period, or an average of 0.049 haemarthroses/year/patient in the prophylaxis period compared to 0.032 in the initial period and 0.070 in the inhibitor period. Five children had no haemarthroses, three had a single episode of haemarthrosis, two prior to the prophylaxis period and one during prophylaxis, one had four episodes, all prior to the prophylaxis and one had ten haemarthrosis, six before prophylaxis and four during prophylaxis.

Table 4. Haemarthrosis and haemorrhagic episodes during each follow-up period.
4. DISCUSSION
The benefits of non-preoperative rFVIIa-mediated secondary prophylaxis in haemophilic patients with inhibitors have recently been supported by reported data [7-11]. Compared with previous on-demand therapy, Konkle et al. found the frequency of bleeds was significantly (p < 0.0001) reduced by 45% for 90 µg/kg rFVIIa and 59% for 270 µg/kg rFVIIa during prophylaxis in 22 patients who had high requirements for on-demand therapy and were given prophylactic treatment daily for three months [12]. The benefits of prophylaxis were prolonged into the post-prophylaxis period when the reduction in bleeding frequency was maintained. However, orthopaedic joint scores did not change during their trial.
As in haemophilia patients without inhibitors, there is a rationale for adopting prophylactic approaches to prevent bleeding in inhibitor patients, affording this group similar opportunities for protection against development of arthropathy [20]. Several early findings suggest that early prophylaxis may be efficacious [11-13]. Early prophylaxis is best characterized as the beginning of preventive treatment with rFVIIa prior to the onset of joint damage, as defined by the European Paediatrics Network for Haemophilia Management (PEDNET), or more broadly by Berntorp et al. [21,22]. Based on these criteria, The Working Group of Experts for the Treatment of Haemophilia Patients with Inhibitors of the SETH created a registry and conducted a retrospective survey among Spanish haemophilia centres on early prophylaxis with rFVIIa in children, aged three years or less, who had haemophilia A and high-titre inhibitors, with no or minimal suspected joint damage. This study included two children with ≤four haemarthroses in the same joint and ≤one in the remaining eight. This registry is currently on-going, collecting prospective data.
Haemorrhages and haemarthoses per year were reduced during the prophylaxis period with rFVIIa. However, it is not justifiable to compare the pre-prophylactic periods with the prophylaxis period because the risk of haemorrhage—particularly haemarthrosis—increases with age, body weight and activity [23]. It is more relevant and interesting to compare our results with those from haemophilia A patients of similar ages without inhibitor who are receiving early prophylaxis with Factor VIII. In the Manco-Johnson series [1], patients had a mean age of 1.6 years, and had previously suffered a mean of 6.2 haemorrhages, but with a longer follow-up period, a mean of 49 months per patient. In the on-demand group, the incidence of haemorrhage was 17.7 per patient/year and the incidence of haemarthroses was 4.9 per patient/ year, while the number of haemorrhages was 3.3 per patient/year and haemarthroses 0.63 per patient/year among patients receiving prophylaxis with FVIII. In our study, incidences were clearly lower, 0.17 haemorrhages per patient/year and 0.049 haemarthroses per patient/year.
It is also important to emphasize that five of our ten patients, after the three recorded periods and after successful ITT, attained tolerance to Factor VIII without target joints and with a minimum number of joint bleeds. One patient had four bleeds in the same joint; three had one joint bleed, and five had no joint bleeds. The remaining patient suffered 10 joint bleeds during this period (four during prophylaxis) as a consequence of the stormy period prior to prophylaxis.
The wide variations in rFVIIa dosages used point to the need for establishing an optimum schedule for prophylaxis. In previous reports, prophylaxis regimens ranged from 200 - 250 µg/kg per week to 90 - 270 µg/kg per day [12], with many patients receiving lower rFVIIa doses than those recommended for on-demand regimens [9]. In our study, children received either 90 µg/kg three times weekly (270 µg/kg per week) or 90 µg/kg daily (630 µg/kg per week), with no differences in outcome. These results suggest that longer dosing intervals may yield similar results in some patients, reducing dose, frequency, overall rFVIIa consumption and, thus, overall cost of the prophylaxis.
A recent ITT study demonstrated that the number of haemarthrosis and bleeding episodes is less when using a high-dose regimen of 200 IU/day of FVIII, rather than lower doses of 50 IU per three times week, even though both regimens achieved immune tolerance [24]. The results of our study suggest that ITT regimens with FVIII at 50 IU/kg three times week, and early prophylaxis with rFVIIa at 90 µg/kg three times/week, produced a good response in four of our patients, indicating this regimen could be a viable alternative to high-dose ITT (FVIII at 200 IU/kg/day) [24]. Therefore, the cost of rFVIIa prophylaxis may not be much more than that of FVIII prophylaxis in non-inhibitor patients, a widely accepted treatment, especially considering that patients with inhibitor after successful ITT will still require prophylaxis doses with FVIII in the future.
The sustained prophylatic effect of rFVIIa is somewhat unexpected from an agent with a relatively short plasma half-life. Recent findings show that FVIIa binding to tissue factor (TF) on the cell surface induces TF endocytosis and mobilizes the translocation of TF to the cell surface from the Golgi, which increases TF expression at the cell surface. This process involves activation of the protease-activated receptor-1 [25,26] and appears to be of greater importance than rFVIIa plasma half-life [27].
In addition to the efficacy of early prophylaxis with rFVIIa, the safety of these new regimens is of paramount importance. No thromboembolic adverse events occurred in our patients. However, six of our ten patients were hospitalized up to twelve times for complications related to catheters implanted to facilitate rFVIIa prophylaxis and ITT. Four of the infected catheter had to be changed.
In summary, although this study has the limitations of being retrospective, not controlled and a short series, bear in mind that patients with inhibitors can have dramatically variable patterns of bleeding from unknown causes, this is the first complete report on early prophylaxis with rFVIIa in high-response inhibitor haemophiliacs. Our results suggest that early prophylaxis with rFVIIa should be considered as highly efficacious as prophylactic FVIII in haemophilic patients without inhibitors. However, the short follow-up period in our series calls for caution when comparing our results with those of other series of haemophilic patients without inhibitors. This would signify that after the risky period of inhibitor, haemophilic patients with eradicated inhibitor will be able to continue FVIII prophylaxis with few or no haemarthroses.


NOTES