Growth of Young Olive Trees: Water Requirements in Relation to Canopy and Root Development ()
1. Introduction
In Tunisia (36.5˚N, 10.2˚E), climate components vary greatly from year to year and between years [1] affecting yield and growth of olive (Olea europaea L.,) orchards, which are growing mostly under rain fed conditions. Olive trees receive annually no more than 300 mm of rainfall, which is mostly confined to the winter season, and no water is supplied during the period of fruit development. As a result, shoot elongation, olive growth and production remain largely dependent on the late winterearly spring soil water reserves. Some studies [2] showed that yield was also correlated to the previous year’s rainfall amount (r = 0.71), which improved shoot elongation and increases the potential sites for olive production, while the seasonal water supplies affected the current year’s production, and particularly, fruit enlargement and oil accumulation. This situation generates fluctuating olive productions, which varied annually from 60,000 to 300,000 tons depending on the events that occur during the growing cycle.
To face this situation, and as Tunisia has to export annually an important amount of olive oil to the EU (47,000 tons), government, through its financial assistance program, tried to encourage farmers to plant olive trees at higher densities and to irrigate their orchards in order to increase and regulate the national production, averaging actually 180,000 tons of olive oil since the 1970’s. However, this needs a good knowledge of the olive tree in general and specifically the physiological processes involved for a judicious use of water.
Most research studies carried out on olive trees in the world [3-9] and in Tunisia [2,10,11], dealing with the biological and physiological processes that occur during the biennial growing cycle of adult trees, in relation to climate, water use, growth and production, showed that most olive cultivars respond favourably and quickly to densification and water supplies. Indeed, when it is properly applied, at suitable amounts and precise stages [12-16], irrigation improves production and fruit size at harvest [17,18], shoot and branch lengths [19-22], trunk diameter and canopy size [8,23-26]. However, the response of the tree may be different from that expected because other factors may interfere like fruit load, root activity and temperature.
Growth of olive tree is a complex phenomenon, governed by exogenous and endogenous factors. The cyclic growth pattern occurs over two growing seasons [5]. Early works [27-29] and recent studies [9,21] reported two main periods of active growth, occurring in the spring before flowering and then in the autumn when wet conditions prevail. However, time and rates may vary according to location and year. Active growth was observed from the spring to early summer in Chania [8], whereas only one active period of growth was observed in regions of high altitudes, over the summer months [6]. In the irrigated orchards, a different growth pattern was reported for cultivar Picholine Marocaine [30], with major growth recorded during the spring period. In autumn, when the temperature decreased, a second flux of growth was reported in most cases [5,6,31].
New buds and shoots grow rapidly in response to the increasing early-spring temperatures [32]. A minimum of 12˚C is required to trigger buds development in the spring with an optimum of 13.8˚C [4,6,33]. The ultimate elongation of buds is well correlated to the average monthly temperature [34]. It depends also on bud position. It was reported that the axillary bud development is always repressed by the terminal growing apices, which are dominant [34]. However, this dominance is influenced by irradiance, soil fertility, water availability, growth regulators and pruning [20,33]. Buds continue their development and provide vigorous shoots if favorable conditions prevail. The seasonal temperature may accelerate or decelerate their growth rates, but it does not modify their cyclic growth pattern, which is highly influenced by the daily absolute temperatures and the seasonal radiant energy accumulation [33-37]. Recent research work [20] showed that early shoot growth is highly correlated to the maximum and minimum daily temperatures but also to root development [7,26] and their ability to extract water and nutrients from the surrounding area. It is also affected by the irrigation method and water distribution. However, fruit load and competition for assimilates between the newly formed shoots and the developing fruits seem to be the most important factors governing growth of adult trees [30,33,34,37-40]. They were evoked and discussed in most papers, showing that following a high fruit load year, fruits compete with shoots and reduce quantitatively their length, the number of nodes and thus the potential sites of flowering and production [7,41]. In recent papers [18,42], authors indicated that after fruit set, shoot development becomes dependent on the available assimilates, which are mostly driven to fruits. At this stage, olives becoming the stronger sinks, attract the nearby available substances as well as for those located in other sites, and so, compete with shoots, but also with roots and flowers buds [3,6]. They can inverse and even inhibit induction of new flower buds, and thus promoting the alternate bearing behaviour [4,7,30,43-45].
Fruits develop rapidly after fruit set following a five stage sigmoid curve, with two latency periods [6,22,35, 42]. Large amounts of nutrients are needed during fruit development to supply the simultaneous growth of olives, shoots, roots and buds [32]. If favorable conditions prevail, high rates were observed during stage I of cell division and stage III of cell enlargement. They are water and temperature dependent [4,6,49]. During stage I, a certain number of cells are provided, which is highly correlated to fruit size at harvest [7,34,46-48]. During Stage II, of pit hardening, the embryo grows rapidly to attain 80% of its full size. Its development depends on water and assimilates-availability. Low root activity lead to low yields and smaller fruits.
Root development influences all the physiological processes of the tree [50]. However, despite of its importance, the root system is possibly the least explored area in crop physiology because of the difficulty involved in reaching it, in addition to the important spacial and temporal variabilities which could generate many constraints to root extension. Early works dealing with this item were made in North Africa on cultivars Chemlali and Picholine Marocaine [51,52]. Studies carried out few years later in Spain, Italy and Tunisia, investigated the relationships between water and root development [26, 53-61]. They show that apart from the genetic factor and the origin of the tree [21], root distribution can be markedly influenced by the neighboring trees and the soil characteristics, mainly soil texture and depth [62]. Also, roots proliferate within the potential root zone regardless to the irrigation method [23,24,63,64]. Localized irrigation increased root length density, but it decreased their spread, largely confining them to the wetted volume and nearby the trunks [7]. Root extension depends also on the available carbohydrate amounts [65] and the phenological growth stage [8]. Rapid growth is observed in spring and autumn and precedes shoot growth. When no competition with other organs for carbohydrates occurred, for example for young olive trees or/and for vigorous trees, important root extension and greater root densities were reported [60]. Adversely, root development may be limited by the previous year’s production. Low carbohydrate resources led trees to reduce their canopy growth and root length and even, could deteriorate the root-canopy ratio as a result of competition between shoots, flowers, fruits and roots [65]. Reduction of root-canopy ratio implies systematically reduction of the capacity of roots to absorb water. In terms of root balance, the importance of the water collecting system resides in its capacity to obtain water to support the transpiring leaf area [24,66]. It can be determined via an estimation of the total root length through a monitoring of root density [20,67]. For olive trees, values ranging between 0.1 and 1.0 cm·cm−3 are reported [24]. They are lower than those provided for herbaceous crops and some deciduous orchards, although the root system of olive trees can be extensive and deep. Such measurements could provide reliable estimates of comparative activities.
As shown in this review, large knowledge is provided for adult olive trees, but little information is available for young plants. Some works [7,33,39], reported that young trees are characterized by rapid growth and important relative rates, allowing them to reach their full size at ten years of age. It was indicated also, that their growth dynamic is influenced by the environmental factors, like water and temperature, but it was not quantitatively analyzed. Such information is important to get because the development of the trees during the first years of cultivation may affect their production performance at later stages.
The present study was carried out in order to get a comprehensive view of the development of olive trees during the first years after plantation through a quantitative analysis of their growth patterns. Growth dynamic was investigated in order to set typical models of growth for young trees cultivated under irrigated conditions. Field measurements were performed since plantation on cultivars Chétoui, Meski, Picholine and Manzanilla, involving the above and underground organs. Studying the root system of young trees Chétoui appeared from our review of prime interest, because without precise information on root distribution, we cannot expect to manage efficiently the olive orchards. When and where roots grow is crucial to understand the functioning of the root system and its relationship with the other parts of the tree. On the basis of canopy and root growth measurements, a methodological model is proposed for determining the irrigation requirements of young olive trees.
2. Materials and Methods
2.1. Site of Experiment
The experiment was carried out in northern Tunisia (36.5˚N, 10.2˚E) at the Research Farm of the National Institute of Agronomy. In this region, the climate is Mediterranean, being dry and hot from May to September with annual average rainfall of 450 mm and reference evapotranspiration approximating 1200 mm. The average minimum temperature of 7˚C is recorded in January and the highest, of 24˚C in July. The autumn and spring seasons are warm, allowing growth over six months-longperiod.
The experiment involved own-self-rooted olive trees of cultivars Chétoui, Meski, Manzanille and Picholine, which were chosen on the basis of their commercial importance [68]. Chétoui and Picholine cultivars have high fruit quality and commercial value. Manzanille cv., is known to be well adapted to high densities. Meski olives are appreciated by Tunisian consumers. Trees were planted at 6 m × 6 m spacing on a textured clay soil (29% C, 49% L, and 23% S), of about 2 m depth. The volumetric soil water content was measured in the laboratory at field capacity (50%) and at the wilting point (26%). Trees were arranged in four plots following north-south orientated rows. The experimental design was a randomised complete block with four replications. Individual plot size was of 112 plants with 7 × 4 trees of each variety.
About crop management practices, all trees were treated equally following the recommended standards, elaborated for Tunisian high density orchards [69,70,72]. Soil was tilled annually in late winter and then in early summer and early autumn. N-fertilizer was applied twice a year in March and September (additional amount of 100 g per tree for each year of growth), while K and P fertilizers were given at planting, only. Mineral nutrient analyses carried out in 1998 and then in 2003 showed satisfactory nutrition levels. Pruning was practiced on the third, fourth and fifth years after planting to a single tree trunk following a classic form with main branches starting at 0.7 m above the ground. Different degrees of severity were applied according to the year and variety. The main tree axis wasn’t pruned. Biomass production (wood from pruning) and wood water contents were determined annually for each cultivar.
Dry conditions prevailed from 1999 to 2002 with annual rainfall ranging between 327 and 440 mm. The year 2001 was the warmest and the driest with only 327 mm of annual rain, amongst 92 mm were received between March and September. For this same period, the minimum and maximum values were recorded in 1999 (60 mm) and in 2003 (346 mm), respectively. Temperatures increased consistently in 2001 and 2002 with annual increases reaching 17% and 16% for the minima and 8% and 7% for the maxima, respectively. High temperatures were also recorded at the beginning of the year 2003, exceeding the cumulative averages by about 300 degreedays, while the summer absolute temperatures ranged between 36˚C and 46˚C, with 27 days of sirocco (high temperature exceeding 35˚C and very low air humidity). Table 1 reports data relative to rainfall amounts and temperatures recorded during the period of experiment.
To avoid the negative effect of high temperatures, irrigation was applied beginning from early spring. It was supplied by furrows (basin and drain) during the four first years and then by a drip system beginning from 2002. A double line per tree row was used with 4 L/h emitters, placed around the tree at 1 m each from the other. Water flows have been programmed with regard to the critical growth stages and water availability. The area wetted during irrigation varied between 1 m2 (1st year) and 6 m2 (6th year).
Crop water needs (ETc) were determined following the formulae developed for the non-standard conditions [74] as: ETc = ETo × Kc × Kr; the crop coefficient Kc ranged between 0.3 and 0.5 following tree age [12], while Kr values were determined experimentally and varied bet tween 0.69 and 0.75. For this purpose, a large white grilled (10 cm/10 cm) sheet was placed below the tree and the shade squares were counted and then compared to the total number of squares (enlightens by the sun and shade by leaves). The obtained percentage was traduced into Kr value. Daily reference evapotranspiration (ETo)
were computed following the Penman Monteith equation [74] and used to establish the irrigation program. Water requirements, irrigation amounts and periods are reported in Table 2.
Water was supplied every year for a 5 - 6 monthslong-period with amounts ranging between 120 and 1740 L/tree/irrigation according to the year. With irrigation and effective rainfall, more than 30% of the crop water requirements were met during the irrigation period. The annual ratios of (I + Pe)/ETc ranged between 0.76 and 1.54.
2.2. Measurements and Data Analysis
2.2.1. Soil Water Content
Volumetric soil water contents (Hv, %) were determined concomitantly with a neutron probe (SOLO 25) which was previously calibrated, and gravimetry at different sites (below the canopy, far from the emitters and along and between the lines of trees), depths (30 cm, 90 cm and 120 cm) and stages of development.
2.2.2. Tree Height, Canopy and Trunk Diameters
Tree height, trunk and canopy diameters were monitored annually beginning from the planting year onward the end of the year 2003. Measurements were performed on 48 trees accounting for 12 trees per variety and per block. Tree height and trunk diameter were measured frequently, while canopy diameter was monitored in September and then, in February before and after pruning in order to estimate the changes of canopy volume. So, two different observations were provided for the same date (the pruning date). For this reason the average curve of canopy diameter was presented separately from that established for the different cultivars.The maximum projected canopy area (Sc) was determined assuming a circular shape

Table 1. Rainfall amounts (mm) and temperatures (˚C) recorded during the period of experiment.

Table 2. Water requirements (ETc, mm) and irrigation amounts (I) computed and applied during the period of experiment for young olive trees aged one to six years.
of the canopy. Wood resulting from pruning was analyzed considering 12 samples for each variety, and water contents were determined before analyzing the dry matter. Shoot measurements were performed on one-year-old lateral shoots of cv., Chétoui following North, South, East and West directions. Leaf area (LA) was determined after pruning by computing the number of leaves on representative branches and their specific area by planimetry [7]. Maximum fruit diameter was monitored from 2000 to 2003 on the same trees. Measurements were performed annually from fruit-set to harvest, considering 5 fruits per tree, i.e., 60 fruits per variety. Mature olives were harvested manually. Production was determined for each tree individually.
Growth rates were determined annually and following the stages of development for each variety. Growth patterns were established for every year of monitoring before proposing an average model for each growth parameter. Relationships between growth parameters were then investigated.
2.2.3. Root Development
Root distribution was studied during the rest period (November-December) on the same Chétoui olive trees aging 1 to 6 years, by extensive observations of their root system. The trench method was used [54,63]. For this purpose, a large pit was made at 40 cm from trunks down to 1.0 - 1.2 m depth. Roots developed at the eastern trench face of the pit were counted and drawn, as well as their diameter by means of a caliper 1/100. Lateral root extension was estimated by measuring the maximum distance of roots to trunk. Total volume of soil occupied by the roots and the explored area were determined assuming a central symmetry to the trunk.
Root densities were determined during the rest period on the same Chétoui olive trees by using the cylinder method [63,67]. A conventional auger was used to take soil samples at 40 cm, 80 cm and 120 cm from trunks, down to 1.0 - 1.2 m depth and following East and South directions (Figure 1). Samples were then washed out abundantly and sieved through a 0.5 mm screen. Extracted roots were counted by adopting a reference scale [67]. Root length was derived from the average root density value for each of the six trees. Details on both protocols are given in Figure 1. The relationship between root and canopy development was examined. The root length/leaf canopy ratio was calculated for each tree.
2.2.4. Crop Water Needs, Canopy and Root Development Relationships
Determination of water requirements following the FAO method is adequate for the standard conditions i.e., when soil coverage reaches 60% and more. But when this area is lower, a reductive coefficient Kr is adopted [74]. In some cases, particularly for young and new orchards, this coefficient may not be precise enough to allow good estimation of the crop water needs. In addition to problems met to estimate Kr values, the Kc is strongly affected by all conditions influencing soil evaporation [75]. Recently, a simple linear relation was proposed between the olive

Figure 1. Protocols elaborated to determine root distribution and root densities of young olive trees aged one to six years.
ground cover (and Leaf Area Index) and the average Kc for the summer months, valid only for ground cover fractions up to 0.25 [76]. For this reason we propose the following methodological approach, or model which is designed to determine the consumptive use of water for young olive trees in relation to their canopy growth and root development during a period where the ground cover and the root system are incompletely developed.
Before full development of the root system, only a fraction of rainfall is accessible to trees. Thus, water balance equation should consider the area concerned by tree transpiration i.e. where roots are active (Sr); Sr is assumed to be circular and increases following a logisticshaped curve as given by the following equation:
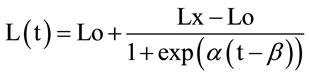
where (t) is the number of years from planting, Lo, Lx dimension of interest respectively at planting and at maximum growth, α, β are adjustment parameters.
On the other hand, and in order to link the water supplied to trees to the evaporative demand, a supply ratio (Ksupply) is defined that takes into account only the treerelated quantities by this equation:

Considering that irrigation (I*, m3) is supplied by localized system or in small basins around the trunk, only a small surface is wetted and affected by soil evaporation and transpiration. Irrigation water is therefore assumed to be fully accessible to the root system of the tree. In another hand, effective rainfall for a single tree (P*) is taken as the volume of rainfall (P) available to the root system which could be approximated by the following equation as:

The evapotranspiration volume of an individual tree (ET*) can be estimated from the root area of the tree as:
 .
.
Different water supply ratios were determined as I/ET0 (irrigation supply), P* + I*/ET* (volumetric total supply) and I*/ET* (volumetric irrigation supply) and compared to Kc-FAO. These ratios were calculated for the period April-August over the first six years of cultivation.
3. Results
3.1. Vegetative Growth
3.1.1. Growth Curves
Tree height, trunk and canopy diameters were analyzed for the six years of monitoring and then for the overall period. The average curves are shown in Figure 2.
Tree height (Figure 2(a)) increased through the six years of cultivation following an S-shaped curve, with
increasing differences between varieties. At the end of the experiment, trees reached an average height of 447 cm, with maximum and minimum values recorded for cultivars Chétoui (472 cm) and Meski (429 cm), respectively.
Trunk diameter grew regularly after planting with increasing rates and differences between varieties to reach 157.9 mm six years later (Figure 2(b)). During the first year, trees presented approximately the same trunk shape and value. It was circular and grey-green colored. Three years later, it became oval and acquired a rough texture. Differences between cultivars increased greatly after the first pruning year, i.e., 2000.
Canopy diameter grew following an S-shaped curve, with increasing differences between cultivars and substantial growth observed annually from April to September. Six years after plantation, an average maximum value of 448 cm was recorded for Picholine, while Chétoui, Meski and Manzanille reached lower diameters and respective values of 424 cm, 402 cm and 393 cm (Figures 2(c) and (d)).
One year old shoots grew following an S-shaped curve with rapid growth occurring from mid of April to end of July (Figure 3) and larger variation between trees during the summer months, resulting from the different crop load. Slow growth was observed after the month of September. Shoots reached at the end of the growing season (2003) an average length of 46.2 cm and an average seasonal increase of 24.9 cm.
3.1.2. Annual Increases
Annual increases varied consistently following the growth parameter, year and variety as shown in Figure 4.
Annual tree height increases (Figure 4(a)) ranged between 21 cm (2003) and 126 cm (2001) following the year. During the planting year (1998), the average gain was 66 cm. In 1999, the value was lower, of about 63 cm. Most growth was recorded during the pruning years, with average values of 77 cm, 126 cm and 87 cm, recorded in 2000, 2001 and 2002, respectively. The same figure

Figure 3. Average growth curve of lateral shoots (cm) recorded during the last year of the experiment made on three olive trees of cultivar Chétoui. Values are means ± standard errors which is represented as vertical bars.
showed some differences between cultivars, although they presented the same growth pattern (Figure 4(a)). The largest variations were recorded in 1998, the planting year and in 2000, the first pruning year. For 1999, the annual gains were 62 cm, 63 cm, 61 cm and 67 cm for cultivars Manzanille, Chétoui, Meski and Picholine, respectively. With regard to the previous year’s growth values, an increase of 4.9% and 13.4% was ensured for cvs., Meski and Picholine, respectively. However, a significant decrease was recorded for cvs., Manzanille and Chétoui, with respective values of 13.8% and 16.0%. This controversial behavior was apparently inherent to the warm conditions and low soil water resources prevailing on that year. Water supplied during the irrigation period, by irrigation and effective rainfall, met only 30% of the crop water needs. During the first pruning year, 2000, tree height gains varied from 68 cm for Manzanille to 91 cm for Chétoui, with an average value of 74 cm,
thus assuming an average increase of about 18.2% with regard to the previous years’ growth values. The largest differences between varieties were recorded on that year, when severe pruning was practiced under warm conditions. Irrigation and effective rainfall met 60% of the annual water needs, with 17.4 mm received in July 2000. Apparently, these conditions enhanced the summer growth and the early autumn development. On the following year, 2001, an important elongation was recorded with annual gains ranging between 121 cm for Meski and 128 cm for cvs., Picholine and Chétoui. Most of this growth was produced during the spring period and apparently, it has been enhanced by the favorable seasonal conditions of high temperatures, monthly rainfall supplies (50 mm), and the moderate pruning which reduced canopy size by 18%, only. Rainfall and irrigation supplies received from April to October (112 mm) sustained the summer growth. In 2002, annual gains ranged between 80 cm for Chétoui and 95 cm for Meski. Most of this growth was produced during the spring season under well watering conditions. About 90% of the crop water needs were met during the irrigation period, with a monthly rainfall amount of 30 mm, received in April and May. The last year of experiment was characterized by low tree height gains reaching no more than 28 cm in average. This growth occurred under water logging, warm and high fruit load conditions. The highest and the lowest gains were recorded for Chétoui and Manzanille cvs., respectively.
During the first year, the trunk diameter grew slowly, providing an annual growth of 13.1 mm (Figure 4(b)). Then, rates increased assuming important gains during the three following years. The peak value of 34.9 mm was recorded on 2001, while the lowest, of 18.5 mm, was recorded on the fifth year. During the last year of experiment, 2003, the trunk diameter assumed an important gain, of 28.8 mm. Differences between varieties increased significantly after the first pruning year (2000), and the largest variations were recorded on the fifth year.
Annual increases of canopy diameter (Figure 4(c)) fluctuated between 15 cm (1998) and 89 cm (2002) depending on year, with large differences between varieties, resulting from pruning. Chétoui cv., grew assuming increasing gains, whereas Picholine, Meski and Manzanille cvs., showed fluctuating rates. As a result, the growth pattern provided for canopy was different from that observed for tree height.
The average curves (Figure 4(d)) showed maximum annual gains for tree height and trunk diameter in 2001, and a year later for canopy. Maximum annual gains were always recorded for Chétoui or/and Picholine while the minimums were observed for Meski and Manznille cvs., except on 2002, where the lowest and the highest values were recorded for Chétoui and Meski cvs., respectively. These major differences indicate that growth may occur differently even when trees are treated similarly, and that besides climate, water and pruning, the cultivar should be considered as another variable that can explain some of these variations.
The severe pruning practiced on 2000, reduced canopy and trunk diameters’ growth during the same year, but it enhanced the development of all parameters during the following years. Abundant new vegetation was, then, produced, promoting flowering, biomass production and yields (3.1, 2.3 and 2.5 tones/ha of olives recorded for cultivars Chétoui, Picholine and Manzanille. But, trunk diameter and tree height growth were reduced consistently, apparently as a result of competition with the growing fruits. On the following year, 2003, canopy and tree height growth were reduced, probably because of the limited potentialities of the trees, which provided high fruit productions for two successive years. High temperatures prevailing during the summer may contribute also to limit the translocation of assimilates to canopy. At the end of the growing season, high amounts of rainfall were received, enhancing substantially the trunk enlargement, while canopy diameter increased by 33%, only.
3.1.3. Growth Dynamic
1) Average patterns Average growth patterns were established on the basis of regular monitoring of tree height, shoot length and trunk diameter and estimation of the daily rates for the six years of the experiment.
Tree height growth pattern (Figure 5) shows sustained growth all over the growing season and even during the summer months and the rest period (quiescence). The observed trends could be linked to seven distinct periods, amongst three periods of rapid growth. The first period of growth or “latency period” (Period 1) was observed during the winter and early spring seasons (DecemberMarch), with average rates ranging between 0.05 cm/day and 0.11 cm/day. Then, rates increased rapidly, beginning from March and marking the second period of the graphic pattern (Period 2), which was identified as the first active period of growth. The highest rates of this pattern were recorded during this period, with a peak value of 0.67 cm/day, recorded by mid of April. Period 3 was observed from mid of April to end of May. It was characterized by low rates (0.14 cm/day). During this period, flowering, fruit set and early fruit growth occurred, beginning from the second year after plantation. Summer growth was observed from the end of May to early September and occurring with variables rates. From end of May to mid of July (Period 4), tree height grew with increasing daily rates, from 0.14 cm to 0.31 cm. The summer peak was observed by mid of July after the well known “June fruit drop”. Then, rates decreased to reach

Figure 5. Growth patterns of tree height and lateral shoots (cm/day) determined for young olive trees during the first six years of cultivation with indication of the successive phenological stages. Seven distinct growth stages were identified for tree height. Period 1: latency (January-March). Period 2: spring active growth (March-mid April). Period 3: early summer slow growth covering flowering, fruit set and early fruit growth. Periods 4 and 5: summer growth and pit hardening period which is observed end of June. Period 6: autumn growth (September-October). Period 7: Quiescence (November-December).
0.21 cm/day by the end of August, characterising the Period 5. The autumn growth (Period 6) occurred with daily average rates ranging between 0.21 cm and 0.29 cm, and peaking by mid of September. Rates decreased after the month of October, progressively to reach their lowest values in December, when entering the quiescence period (Period 7).
Shoot growth pattern showed also seven distinct periods of growth (Figure 5), with maximum values observed during the two last weeks of April. This means that the peak value of tree height preceded that of shoots. Summer growth was observed in July, while the autumn growth covered the months of September and October with average rates of 0.12 cm/day and 0.05 cm/day, respectively. Then, rates decreased gradually to reach their lower values during the rest period.
Trunk diameter growth pattern was different from that observed for tree height (Figure 6). Trunk grew with sustained rates all over the year, with values ranging between 0.10 mm/day and 0.03 mm/day. Most trunkenlargement occurred during the spring-summer period, with a peak value recorded by the end of July.
Tree height and trunk growths interfered during the growing season, showing contrasted rates during the

Figure 6. Average growth pattern of trunk diameter (mm/ day) determined for young olive trees during their first six years of cultivation andcompared with the tree height growth pattern.
summer (July) months. Maximum trunk growth was observed in summer due to cambium activity. Slow growth was recorded for tree height during this same period.
2) Variations of the average tree height pattern Seasonal variations instead of year’s variations: The winter-spring growth produced in average 49% of the annual growth, while the summer period assumed 39% of the annual gain. Autumn growth contributed with 12%, with large differences between years. Indeed, time, duration and rates of the different periods of growth varied in a wide range depending on the prevailing year’s conditions as shown in Figure 7.
The shortest spring period of less than one week was recorded in 2002 and the longest in 2003 (Figure 7(a)). The summer growth period was lengther with duration variying from 20 days to 50 days. In 2003 the summer growth wave was recorded later but this didn’t affect the autum growth period which was observed at the same time i.e., in October. The longest summer growth period was recorded in 1998.
Rates varied also consistently from year to year (Figure 7(b)). The highest rates were recorded in 2001 and the lowest in 2003. The three growth waves identified in the average model were not observed on each year of monitoring. They were absent in 2003.
Variety: Differences between varieties were observed mainly from May to end of September. Chétoui and Meski cultivars provided the highest growth rates from
 (a)
(a) (b)
(b)
Figure 7. (a) Average duration and time of the different tree height growing periods observed during the six years of experiment (1998-2003) with indication of the average seven distinct periods of the average graphic pattern; (b) Seasonal rates observed for tree height during the different years of monitoring.
April to mid of July. The cultivar Picholine sustained low rates during this period. Manzanille cv., presented some growth during the autumn season and low rates from January to end of March (Figure 8).
All varieties produced low rates from mid of July to mid of August. This period is known as the flower bud induction period. The low rates recorded in 2002 and 2003 resulted apparently from the presence of fruit interference, since high yields were obtained for most varieties on those years. Significant summer growth was observed in 1999, 2000 and 2001 under variable watering conditions, but low fruit load.
3.2. Fruit Development
Olives grew from May to November with variable rates for about 200 days-long-period (Figure 9), showing different growth stages with rapid growth occurring mostly from May to August and differences between cultivars. The lowest values were recorded for Chétoui cv., and the highest for cv., Meski. The largest differences were observed during the summer period.
The average growth pattern established for olives (2000-2003) showed different periods of growth which are summarized in Table 3.
The first stage (Stage 1) of growth occurred from mid April to end of May for 20-25 days-long-period, covering flowering and fruit-set. During this period, fruits grew rapidly with average daily rates ranging between 0.14 mm and 0.99 mm. This period was followed by stage 2, which covered about 48 - 49 days-and occurring from end of May to mid of July with average daily rate of 1.43 mm. It was the first active stage of olive growth. Few days after the beginning of this stage, a large number of flowers and fruits dropped. The third stage of fruit development (Stage 3) was that of pit hardening, occurring with

Figure 8. Average growth pattern of tree height (cm/day) observed for cultivars, Manzanille, Chétoui, Meski and Picholine.

Figure 9. Growth curve for Manzanille, Chétoui, Picholine and Meski olives (mm). Each value is average of 120 olives. Values are means ± standard errors which is represented as vertical bars.

Table 3. Average growth stages of olives observed during the years of monitoring (2000-2003).
average rates ranging between 0.50 mm/day and 0.63 mm/day. This event happened during the two last weeks of July, i.e. about ten weeks after full bloom. Olive stones solidified during this stage, which was generally characterized by the lowest rates of growth for both olive and vegetative growth. This process may be prolonged to 10 August as it was observed on year 2002. The fourth stage (Stage 4) was observed just after pit hardening. It was characterized by slow/high growth rates. It begun generally by the end of July and may continue onward the autumn season as it happened on 2002. Low rates recorded during this period characterized the presumed “flower bud induction process” which may be considered as a separate stage (Stage 5), occurring for 13 - 30 dayslong-period according to the year. Early autumn season was characterized by rapid vegetative and fruit growth (Stage 6) with daily rates ranging between 0.69 mm and 3.95 mm for olives. Fruit maturation was observed between early September and end of November (Stage 7). Olives ensured during this period a certain growth with daily rates varying between 0.75 mm and 1.37 mm. Fruits were harvested between end of October and end of November according to the year. Low growth occurred during this period, with rates varying from 0.51 mm/day to 0.63 mm/day.
3.3. Interferences between Fruit Development and Vegetative Growth
The average fruit growth pattern is presented in Figure 10 and compared to that of tree height.
The average growth patterns of tree height and fruit growth established for the period 2000-2003 (Figure 10), showed low vegetative growth rates during the periods of flowering, fruit-set, early fruit growth, pit hardening, fruit enlargement and maturation. Tree height grew rapidly during early spring. Then rates decreased as flowers develop as a result of competition for nutrients. Flowering was achieved by end of May while olives begun to grow rapidly reaching their peak value, exactly when tree height attain one of its lowest rates. Maximum fruit growth was observed just after the spring rapid tree height growth period, known as the spring growth wave. Their growth rates decreased after this period and fruits grew during the summer and autumn tree height growth waves with lower rates. Then, rates decreased substantially for both parameters to allow “induction of new buds”. However, the year 2000 was different. Maximum tree height growth was observed later in the season, during the two last weeks of April-early of May and coincided with the peak fruit values. In 2003 vegetative growth was sustained at low rates all over the year while maximum fruit rates were reached by early June.
Fruit and tree height interfered also with shoots (Figure 10). Shoot growth sustained high rates during the two last weeks of April, i.e., two weeks after the peak value of tree height, as a result of competition between the terminal apices which ensured the tree height growth (dominant organ) and the lateral buds, which were “momentally” repressed. After this first period of increasing lateral shoot growth, rates decreased gradually as a result of fruit interference. Indeed, the newly developed fruits interfered at this stage with shoots and repressed their elongation.
Growth patterns examined separately for every year of monitoring showed that tree height interfered strongly with fruit development and particularly during the years of high fruit productions (Figure 11). In 2002, most growth occurred in the spring and very low increase was provided during the following seasons. In 2003, growth waves were absent for tree height.
These figures showed also, that the previous enumerated fruit stages (Table 3) may not be observed every

Figure 10. Average fruit growth pattern (mm/day) established for the period 2000-2003 and compared to that of tree height (cm/day). Slow vegetative growth coincided with rapid fruit development.
year. Their length and the rates they achieved varied from year to year. Daily rates varied from 0.26 mm to 1.06 mm in 2000, from 0.31 mm to 3.15 mm in 2001 and from 0.14 mm to 1.77 mm in 2002. The observed stage of pit hardening and the presumed stage of bud induction may or not be separated by a period of rapid growth. On 2002, pit hardening occurred for 30 days-long-period while its duration was only of 10 days on 2000.
Interferences between the vegetative growth parameters and fruits were well illustrated when the growth patterns of productive (2000-2003) and non-productive years (1998-1999) are established. Figure 12 showed consistent differences between the different models. Higher rates were observed for the productive years’ growth pattern. This result was uninspected because fruits are known as strong sinks, driving most of the produced assimilates in order to ensure their development and thus, strongly competes with the vegetative growth, which may be reduced under limited carbohydrate amounts.
Table 4 summarizes the previous results relative to fruit and vegetative growth dynamics with indication of the seven distinct periods of growth.