Analcime-bearing rocks (Komi Republic, Russia) are perspective raw for geological materials. The article represents possibilities of their application for water purification, as raw for ceramic industry and poor ore for aluminum industry.
1. Introduction
Analcime-bearing rocks (ABR) are wide spread in the Timan region (Komi Republic). By the second half of last century the Timan zeolite-bearing province was determined with the area about 150,000 km2 [1]. The Koinskaya zeolite-bearing area is the most studied, where 10 occurrences of ABR were revealed.
We have studied physical and chemical properties of this raw [2]. The conducted researches resulted in possible directions of use of the rocks as sorbent, raw for ceramic industry and poor ore for aluminum industry.
2. Materials
ABR are represented by Upper Permian siltstones and argillites and rare marls. The analysis of mineral composition showed that ABR are characterized by the high content of clay component (50% - 70%), which is saturated by iron oxides and hydroxides. Also quartz (10% - 30%), analcime (1% - 30%), feldspars (2% - 10%), carbonates (2% - 5%), pyroclastic material are present.
In the rocks analcime occurs as isometric crystals, microoolitic concretions, microgeode aggregates or cryptocrystalline cement. Analcime aggregates inlay roundish and oval cavities, and also fill microcracks in the rocks. Different stages of filling of voids by analcime are traced - from insignificant incrustation to complete filling.
The following components are determined chemically (mass.%): SiO2—54-72, TiO2—0.6-1.1, Al2O3—11-18, Fe2O3—3-8, FeO—0.2-0.9, MnO—0.05-0.1, MgO— 0.5-2.3, CaO—0.6-2.5, Na2O—1.4-4.3, K2O—1.4-2.2, CO2—0-0.4, losses on ignition—6-9.
3. Methods
3.1 Preparation of Sorption Material
The researches on purification of potable water of Vychegda River were conducted. The effect of various kinds of filtering load (ABR with size –3.0 + 1.0 mm and quartz sand) on water treatment process was compared. Comparison of ABR was conducted with quartz sand, not with other sorbents (zeolites), because now quartz sand is used at a local wastewater treatment plant. At the use of ABR without any treatment the permanganate oxidability increased from 3.77 to 9.49 mg/dm3. Therefore further researches were conducted on modified rock, obtained by sintering at temperature 500˚C within 1 hour. Thermal treatment allowed removing organic substances and increasing sorption properties. The obtained samples were tested on chromaticity, turbidity, iron content and permanganate oxidability.
3.2 Production of Ceramic Material and Study of Its Properties
Two types of samples were studied: with analcime from 1 to 5 mm (sample 1) and “fine-grained”—sizes of analcime inclusions less 1 mm (sample 2). The samples were prepared by the standard technological scheme. Sintering was made at temperature from 850˚C to 1250˚C with step 50˚C. Heat rate –200˚C/hour. Exposure at the maximum temperature –2 hours. Cooling occurred in disconnected furnace [3].
Standard characteristics of obtained samples of ceramics were measured: water absorption (W) and open porosity (P), flexural strength (σ), apparent density (r) and linear shrinkage (g).
The properties of the ceramic samples were studied by XFA, DTA, magnetic susceptibility, ultrasonic and acoustic analysis.
3.3 Aluminum Extraction
Dealuminization of analcime and ABR was carried out at room temperature by means of hydrochloric and sulfuric acid of various concentration (2%, 3%, 4%, 5%, 7% and conc.), varied processing time (from 5 minutes to 5 days) and grain size (–0.05 and –0.4 + 0.2 mm).
Chemical composition of the initial and treated material was defined by means of X-ray fluorescent analysis (MESA500W, Horiba). This analysis is an approximate-quantitative analysis without considering Na2O and losses of ignition.
4. Results and Discussion
4.1 Water Purification
Figures 1-4 show the results of water purification by quartz sand and ABR. Both kinds of filtering load approximately equally improve organoleptic indexes of quality of water (i.e. chromaticity and turbidity decrease). However the ABR much better reduce iron content in water and lower permanganate oxidability [4].
At the choice of post-treatment method of sewage from boiler-houses of Vuktyl gas-processing facility the technological scheme with ABR was considered the most rational for grain load of filter and membrane installation for water desalting [5]. It is proved that purification of waters with the help of ABR is more efficient by many parameters: sorption of iron ions 0.13 mg-eqv/100g, amines 10 mg-eqv/100g, magnesium 5.94 mg-eqv/100g, phosphates 1.42 mg-eqv/100 g. High adsorptive properties are predicted in relation to oil products and PVA.
The high absorption capacity of uranium (18.77 mg/g) and sorption durability were determined [6]: distilled water desorbed 1.5% radio nuclide, 1 M HCl—11.5%, 1 M CH3COONH4—31.2%. ABR sorb from fluid phase 94.0% - 96.4% radium.
4.2 Characteristics of Ceramic Material
4.2.1. Behavior at Heating
As is shown in Figure 5, sintered samples of ceramics represent quartz, feldspars and hematite. In the samples, sintered at 850˚C, there are no diffraction lines of analcime and sterrite. These crystal phases passed to amor-

Figure 1. Turbidity of water after purification by ABR and quartz sand.
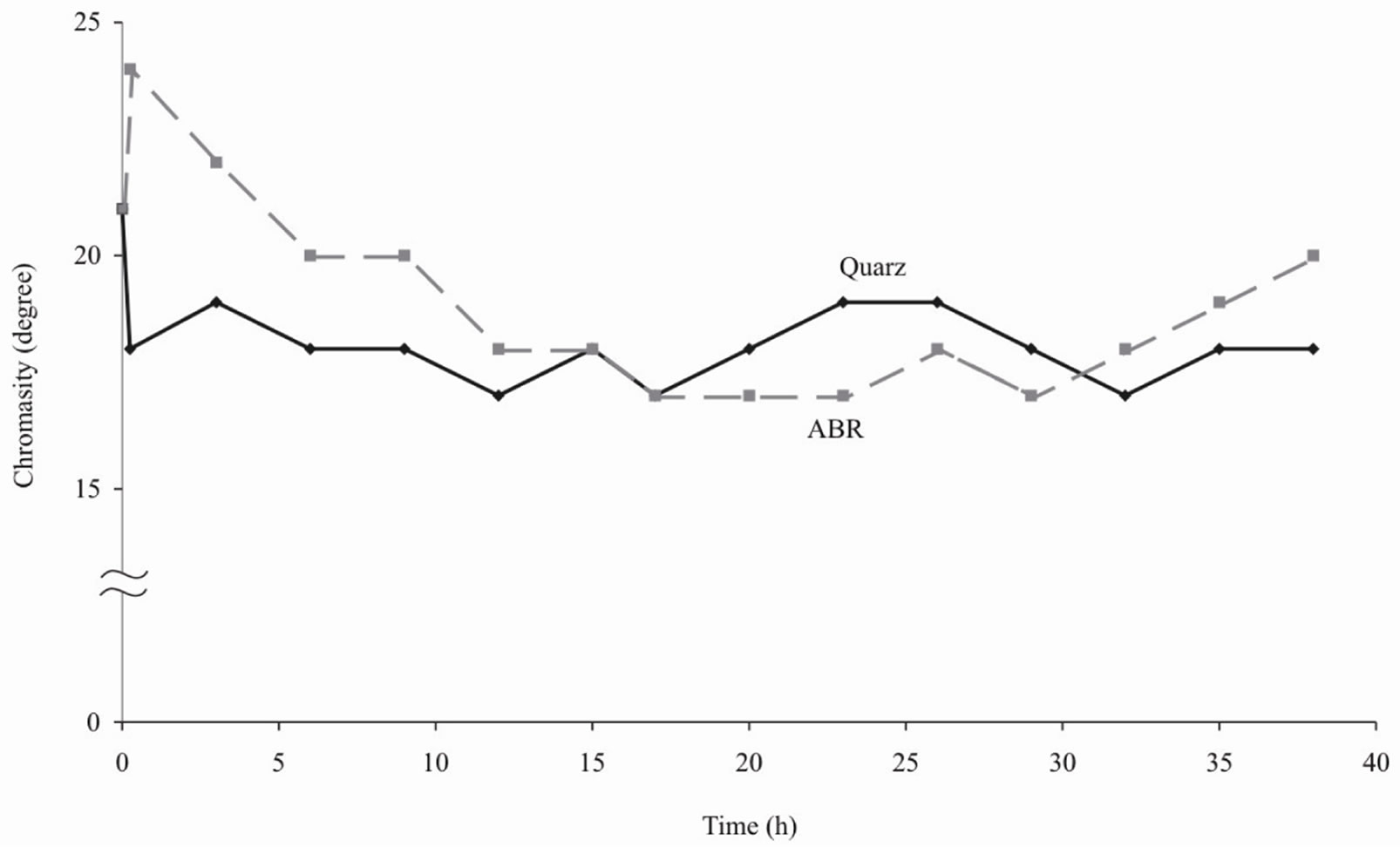
Figure 2. Chromaticity of water after purification by ABR and quartz sand.

Figure 3. Iron content of water after purification by ABR and quartz sand.

Figure 4. Permanganate oxidability of water after purification by ABR and quartz sand.
phous state.
In the range 900˚C - 1000˚C the relative intensity of quartz variates slightly. In sample 2 absolute intensities of lines of quartz were 2 - 3 times more than in sample 1; at higher temperatures the considerable part of quartz passes to glass phase. The relative lines of feldspars monotonously increase with growth of burning temperature, the absolute intensity of their lines in sample 2 are approximately 1.5 - 2 times less in comparison with sample 1. As the absolute intensities of diffraction lines can be generally considered as a measure of quantity of crystal phases in sample, hence, sample 2 contains more quartz and less analcime. Besides, relative intensities of hematite lines increase with increasing burning temperature.
In the range of temperatures 1050˚C - 1250˚C almost complete transition of quartz and feldspars to glass phase is observed at conservation of hematite lines and increase of their intensity.
4.2.2. Water Absorption, Open Porosity, Flexural Strength and Apparent Density
The conducted experiments showed the following. Firstly, the best interval of sintering was determined—about 900˚C, at that sample 2 had higher hardness in narrow interval of sintering (Figure 6). Secondly, during sintering sample 2 was condensed faster, than sample 1 with formation of almost nonporous material. At the same time sample 1 kept open porosity. Comparing the value of porosity with water absorption (Figure 7) it is possible to draw a conclusion on the ability of molecules of water to enter the material structure, i.e. sample 1 is capable to swell. The surface of sample 2, unlike sample 1, was almost completely vitrified that interferes with infiltration of molecules of water into the material structure. Linear shrinkage appeared proportional to apparent density (Figure 8).
4.2.3. Acoustic Properties
Procedures of research of acoustic, electrical and magnetic properties of samples of the received ceramics differ by high rate, accuracy of measurements and are inherently indestructible methods, yielding much richer information on studied objects. Measurements of sound rate (V) yield the information on porosity, hardness and elasticity of the material. Figure 9 shows dependences of V and ultimate flexure strength σ of ceramic materials from temperature of their sintering. Such data allows to judge about reasons of destruction of the sample. If there are fractures, on which the ceramics is destroyed, the dependences V (T) and σ (T) should correlate. Fractures slightly affect ultrasonic sound rate, because the delay of acoustic impulse on a fracture is insignificant. At the same time fractures result in decreasing flexural strength. The obtained data (Figure 5) testify to fracture initiation at burning temperatures above 1000˚C, because ultrasonic sound rate changed slightly, but hardness decreased at 3 times.
4.2.4. Electrical Properties
Figure 10 presents measurements of electrical properties: dielectric inductivity (ε) and tangent of dielectric losses (tan δ), made at frequencies 1 kHz and 1 MHz. At frequency 1 kHz the material polarization can be affected by free ions in ceramics due to its underburning or impurities, for example, adsorption of water vapor. At frequency 1 MHz ion effect is not displayed by measure-