Adsorption of Fluoride from Aqueous Solution Using Low-Cost Bentonite/Chitosan Beads ()
1. Introduction
Adsorption technology is an efficient method for fluoride removal from water and has been widely studied [1]. Recent research work has been devoted to develop lowcost adsorbents to enhance the cost effectiveness for defluoridation. For example, chitin composite [2], modified chitosan beads [3], synthetic apatites [4] and bentonite clay [5] have been demonstrated effective adsorbents for fluoride adsorption.
Bentonite is a common group of clay minerals, which is a hydrous aluminium silicate, and it has been reported as an economical material for adsorption of fluoride from water. However, the low adsorption capacity is the major problem for its possible application. Therefore, it is important to modify the bare bentonite to overcome its drawbacks for fluoride removal. Chitosan, also named poly(β- 1-4)-2-amino-2-deoxy-d-glucopyranose, is prepared from chitin by partial or total deacetylating. Chitosan is a biopolymer which has been cited as an excellent material for defluoridation from water. However, raw chitosan used in the form of flakes or powder is unstable and the adsorption capacity is minimum [6]. Thus, it is necessary to modify chitosan physically or chemically in order to improve its practical use.
The present study deals with the bentonite and chitosan with crosslinker to form an inexpensive and efficient adsorbent. Adsorption equilibrium experiments were used to investigate the adsorption behavior of bentonite/chitosan beads. The effect of various parameters such as bentonite dosage, pH, temperature, initial fluoride concentration and contact time on adsorption capacity of bentonite/chitosan beads was studied. Freundlich isotherm model and Pseudo-second order kinetic model were employed to understand the adsorption process. SEM, EDAX and FTIR analysis were used to characterize the bentonite/chitosan beads before and after fluoride adsorbed. The regeneration experiments were performed to check the reusability of bentonite/chitosan beads for defluoridation.
2. Materials and Methods
2.1. Materials
All reagents were of analytical grade. Bentonite was supplied by Liuhe Group Co. Ltd. (Qingdao, China). Bentonite was used after activated with hydrochloric acid and calcined in muffle furnace at 500˚C. Chitosan (MW 5.1 × 105 Da and degree of deacetylation 95.4%) was purchased from Shandong Hecreat marine bio-tech Co. Ltd. (Qingdao, China). Fluoride stock solution of 100 mg/L was prepared using anhydrous sodium fluoride. Working fluoride solutions of required concentrations were prepared by appropriate dilution of the stock solution.
2.2. Preparation of Adsorbent
The bentonite/chitosan beads was prepared using inverse suspension polymerization [7]. The procedure was: 10.0 g chitosan and bentonite of required quantities namely 1.0, 2.0 and 3.0 g were dissolved respectively in 200 mL acetic acid solution (2%, v/v) for 24 h. Liquid paraffin was added to form a dispersion phase at 50˚C, stirring for 10 min. Then, the system was added with formaldehyde and epichlorohydrin as crosslinker. After that, the beads was washed with petroleum ether, ethanol and deionized distilled water. The wet adsorbent was dried in a vacuum oven at 50˚C. Chitosan beads was prepared without bentonite and served as control.
2.3. Adsorption Experiments
Adsorption experiments were carried out with 50.0 mL of 10 mg/L fluoride solution at a constant speed of 160 rpm at 30˚C for 480 min. Adsorbent of 0.1 g was added to the fluoride solution. The adsorption of fluoride onto adsorbent was studied under different conditions including pH, temperature, contact time and initial fluoride concentration. After adsorption equilibration, fluoride concentration was measured using a fluoride ion selective electrode (PF-1, Shanghai Leici, China). The amount of fluoride adsorbed was calculated from following equation.
 (1)
(1)
where qe is the adsorption capacity of the adsorbent (mg/g) at equilibrium; c0 and ce are the initial and equilibrium fluoride concentrations (mg/L), respectively; V is the volume of solution (L) and W is the mass of adsorbent (g).
2.4. Characterization of Adsorbent
The surface microstructure of the adsorbent was observed with a scanning electron microscope (SEM) and the element composition before and after fluoride adsorption was determined by energy dispersive X-ray analysis (EDAX). In order to confirm the functional groups present in the adsorbent, FTIR spectra of chitosan, bentonite, chitosan beads and bentonite/chitosan beads were recorded on Nexus 470 FTIR spectrometer. The KBr pelleted samples were scanned over the wavenumber range 400 - 4000 cm−1 with resolution of 4 cm−1.
2.5. Regeneration Studies
Regeneration studies were conducted using sodium hydroxide aqueous solution. The exhausted bentonite/chitosan beads was retrieved in 0.5 M NaOH for 12 h and was washed with deionized distilled water followed by drying in oven at 50˚C. The regenerated adsorbent was reused in the next five adsorption experiments.
3. Results and Discussion
3.1. Effect of Bentonite Dosage on Adsorption
In order to optimize the optimal dosage for bringing down fluoride concentration from aqueous solution, bentonite/chitosan beads with 1.0 g, 2.0 g and 3.0 g bentonite and raw chitosan beads were used to adsorb fluoride under identical experimental conditions. It can be seen from the results in Figure 1 that the dosage of bentonite in adsorbent significantly influenced the adsorption capacity of adsorbent. Bentonite/chitosan beads with bentonite dosage of 3.0 g possessed an adsorption capacity of 0.895 mg/g, whereas bentonite/chitosan beads with 2.0 g, 1.0 g and chitosan beads had 0.687 mg/g, 0.528 mg/g and 0.359 mg/g, respectively. Hence, 3.0 g of bentonite was fixed as the optimal dosage in all subsequent experiments.
3.2. Effect of pH on Adsorption
The adsorption data obtained for the effect of different pH values namely 3, 5, 7 and 9 on defluoridation were shown in Figure 2. The maximum adsorption capacity of 1.164 mg/g was observed at pH value of 5, however the adsorption capacity declined with the increasing of pH value. Figure 2 also reflected that the acidic pH could offer more adsorption sites on bentonite/chitosan beads for fluoride removal but the alkaline pH was undesirable

Figure 1. Effect of bentonite dosage on adsorption capacity of adsorbent for fluoride.

Figure 2. Effect of pH on adsorption capacity of adsorbent for fluoride.
for adsorption process for fluoride.
3.3. Freundlich Isotherm Model
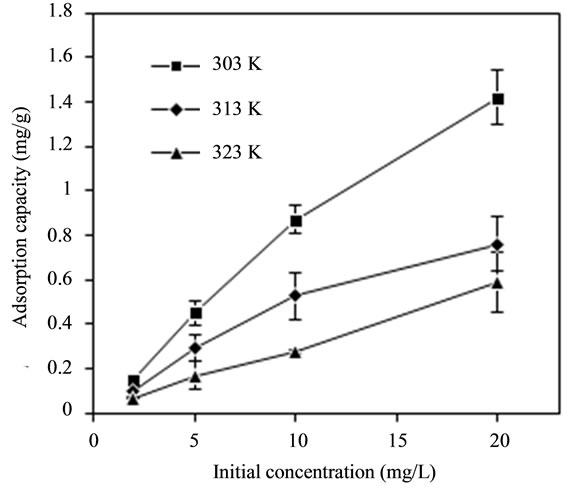
The effect of temperature (303 K, 313 K and 323 K) and initial fluoride concentration (2 mg/L, 5 mg/L, 10 mg/L and 20 mg/L) on adsorption capacity of adsorbent was given in Figure 3(a). The adsorption capacity of bentonite/chitosan beads went up to 1.575 mg/g at 303 K with initial fluoride concentration of 20 mg/L. Apparently, the adsorption capacity of adsorbent declined with the rise in temperature, which is perhaps because the high temperature resulted in the unstability of bentonite/ chitosan beads. Figure 3(a) also indicated the initial fluoride concentration could enhance the adsorption of fluoride onto adsorbent.
Freundlich isotherm is a commonly used isotherm model to analyze adsorption data. Freundlich isotherm model can be written as:
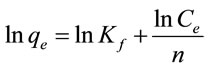 (2)
(2)
where qe is the adsorption capacity of adsorbent (mg/g) at equilibrium and ce is the fluoride concentration in the equilibrium solution (mg/L). The Freundlich constant Kf (mg/g) (L/mg)1/n characterizes the adsorption capacity of adsorbent, and the Freundlich constant 1/n reflects the adsorption intensity of adsorbent.
The fitting curve of Freundlich adsorption isotherm for fluoride adsorption using bentonite/chitosan beads was shown in Figure 3(b) and the Freundlich parameters calculated from fitting curve of Freundlich model were listed in Table 1. The correlation coefficients (R2) values showed that the adsorption data could be well described by Freundlich isotherm model, which suggested the bentonite/chitosan beads was a heterogeneous adsorbent. The magnitudes of 1/n lying between 0 and 1 suggested
 (a)
(a)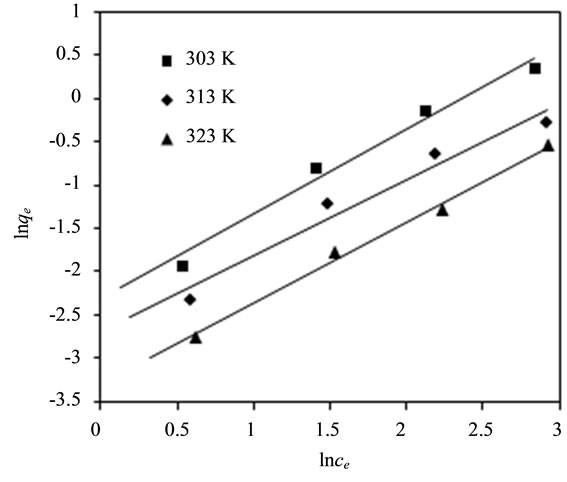 (b)
(b)
Figure 3. (a) Effect of temperature and initial fluoride concentration on adsorption capacity of adsorbent; and (b) Fitting curve of Freundlich adsorption isotherm of adsorbent.

Table 1. Freundlich isotherm parameters of adsorbent.
the adsorption of fluoride onto bentonite/chitosan beads was favourable.
3.4. Pseudo-Second Order Kinetic Model
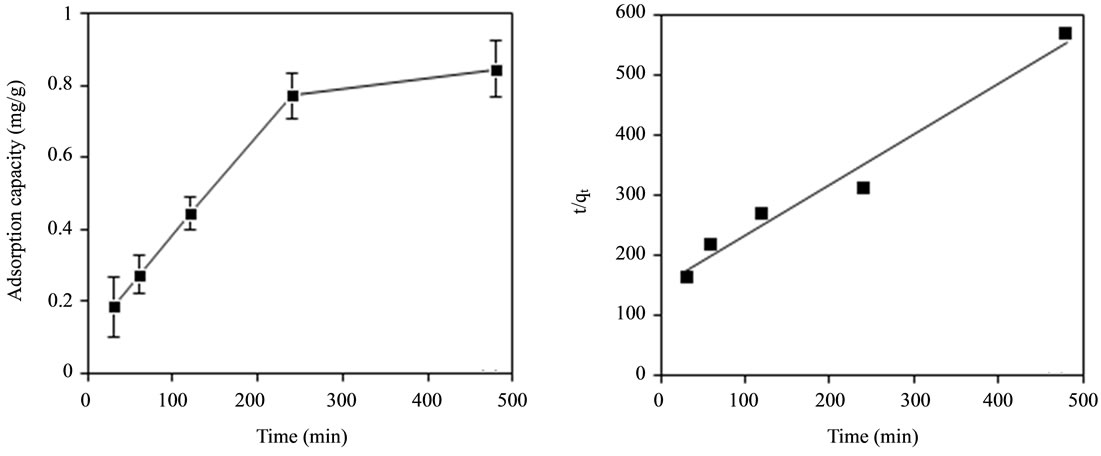
The effect of contact time (30 min, 60 min, 120 min, 240 min and 480 min) on adsorption of fluoride using bentonite/chitosan beads was shown in Figure 4(a). The adsorption capacity of adsorbent increased with increasing time and finally reached saturation at around 480 min.
Pseudo-second order kinetic assumes that chemisorption control the adsorption rate [8]. In order to gain the adsorption behavior through the whole range of process, pseudo-second order kinetic model was employed to analyze the adsorption data. The linear form of pseudosecond order kinetic can be expressed as:
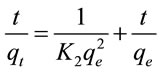 (3)
(3)
where t is the contact time (min). qt and qe are the amounts of fluoride adsorbed (mg/g) at arbitary time t and at equilibrium, respectively. K2 is the adsorption rate constant (g/mg min).
The plots for pseudo-second order kinetic model of adsorbent was shown in Figure 4(b). The correlation coefficients (R2) value was larger than 0.9 (Table 2) which suggested the adsorption followed pseudo-second order kinetic model. Thus, the adsorption process of bentonite/chitosan beads for fluoride was governed by both physisorption and chemisorption.
3.5. Characterization
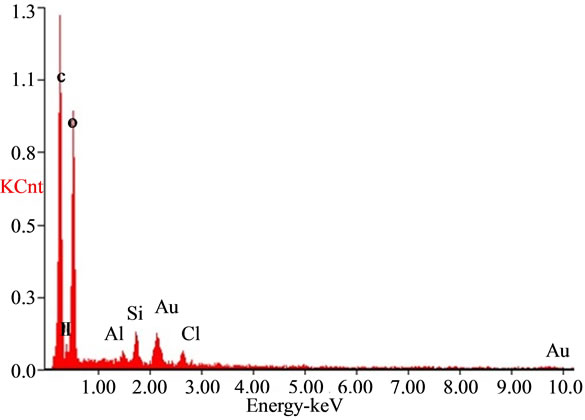
The SEM analysis graph (Figure 5) showed that the sizes of bentonite/chitosan beads were between 50 um and 150 um. The holes on the surface of beads could increase the adsorption sites. The EDAX spectra of the adsorbent (Figure 6(a)) showed the presence of elements in bentonite/chitosan beads. The emergence of fluoride peak in fluoride treated beads (Figure 6(b)) demonstrated the adsorption of fluoride onto adsorbent.
The FTIR spectra of chitosan, bentonite, chitosan beads and bentonite/chitosan beads were compared in Figure 7. The broad band at the region of 3500 - 3200 cm−1 is the overlapping peak of -NH2 and -OH stretching vibrations [9]. As for chitosan beads (Figure 7(c)), the band at 2925 cm−1 and 2884 cm−1 was the characteristic of -CH3 and -CH2 stretching vibrations, respectively. The adsorption peak at 1649 cm−1 assigned to -NH2 bending vibration. The absorbance peak of symmetric stretching of -CO on C3 appeared at 1030 cm−1 suggested that -OH on C3 reacted with crosslinker to form chitosan beads [10]. The FTIR spectrum of bentonite/chitosan beads was given in Figure 7(d), which presented the characteristic of chitosan (Figure 7(a)) and bentonite (Figure 7(b)). Bands at 1399 cm−1 and 519 cm−1 were the -Si-Ostretching and bending vibration, respectively [11]. The FTIR spectra confirmed the successful synthesis of bentonite/chitosan beads.
3.6. Regeneration of Adsorbent
The results of regeneration studies shown in Figure 8 indicated the adsorption capacity of adsorbent decreased from 0.92 mg/g to 0.69 mg/g with 25% loss in five
 (a) (b)
(a) (b)
Figure 4. (a) Effect of contact time on adsorption capacity of adsorbent for fluoride; and (b) Plots for pseudo-second order kinetic model of adsorbent.

Table 2. Pseudo-second order kinetic parameters of adsorbent.
 (a)
(a)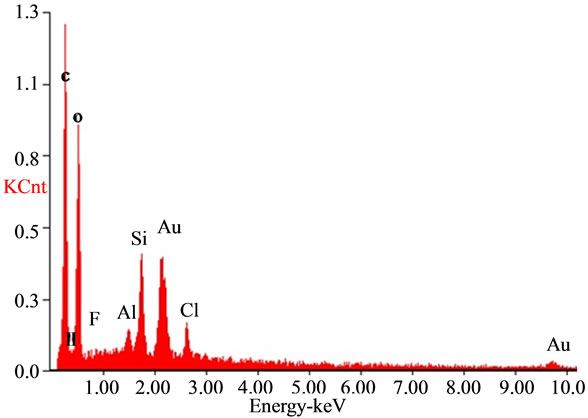 (b)
(b)
Figure 6. EDAX spectra of (a) adsorbent and (b) fluoride treated adsorbent.
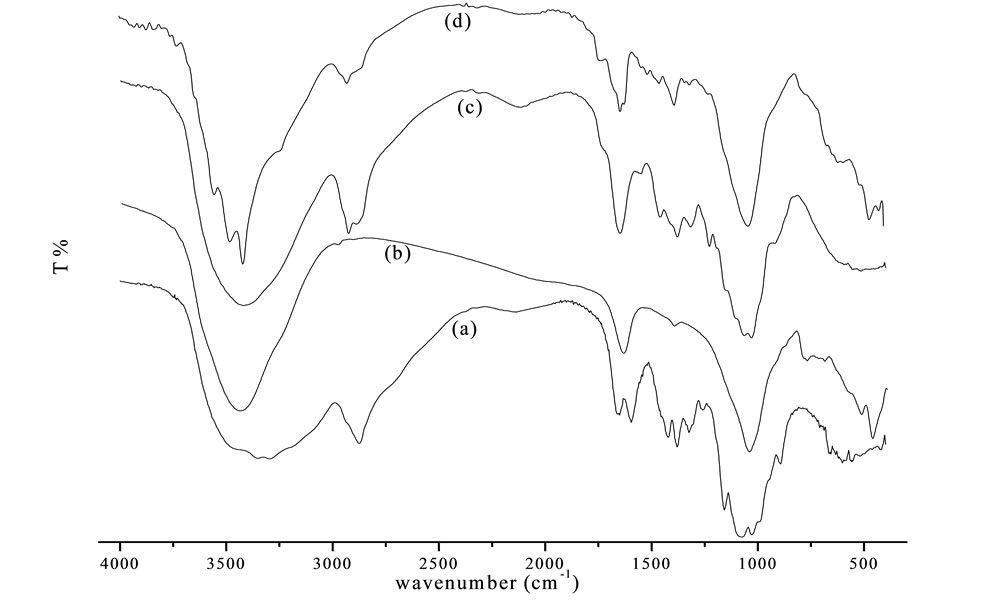
Figure 7. FTIR spectra of (a) Chitosan; (b) Bentonite; (c) Chitosan beads; (d) Bentonite/chitosan beads.

Figure 8. Relationship between reuse cycles and adsorption capacity of adsorbent.
successive adsorption experiments. Therefore, bentonite/ chitosan beads had excellent regeneration ability.
4. Conclusions
1) The low-cost bentonite/chitosan beads were successfully prepared for adsorption of fluoride from aqueous solution using inverse suspension polymerization.
2) The optimal dosage of bentonite was 3.0 g with adsorption capacity of 0.895 mg/g. The optimal pH value for fluoride adsorption was pH 5 where the adsorption capacity of adsorbent was 1.164 mg/g.
3) The adsorption followed Freundlich isotherm model and pseudo-second order kinetic model.
4) Bentonite/chitosan beads were a low-cost, effective and reusable adsorbent for adsorption of fluoride.
5. Acknowledgements
The authors are thankful for the financial support by National Special Fund for Scientific Research on Public Causes (201005020), P.R. China and National Science Foundation of China (31101330).
NOTES