Adaptive Neuro-Fuzzy Logic System for Heavy Metal Sorption in Aquatic Environments ()
1. Introduction
The release of heavy metals from industries into the environment has resulted in many problems for both human health and aquatic ecosystems [1,2]. Heavy metals released into the environment by technological activities tend to persist indefinitely, circulating and eventually accumulating throughout the food chain, becoming a serious threat to the environment [3]. The presence of heavy metals in the environment is of major concern because of their toxicity, bio-accumulating tendency, threat to human life and the environment [4,5]. Lead, cadmium and mercury are examples of heavy metals that have been classified as priority pollutants by the U.S Environmental protection Agency (US EPA) [6]. Various biomaterials have been examined for their biosorptive properties and different types of biomass have shown levels of metal uptake [7]. Tables 1 and 2 show examples of biomass ability to sorb heavy metals.
In recent years, applying biotechnology in controlling and removing metal pollution has been paid much attention, and gradually becomes hot topic in the field of metal pollution control because of its potential application. Alternative process is biosorption, which utilizes various certain natural materials of biological origin, including bacteria, fungi, yeast, algae, plant, etc. These biosorbents possess metal-buffering property and can be used to decrease the concentration of heavy metal ions in solution [8]. A large quantity of materials has been investigated as biosorbents for the removal of metals extensively. The tested biosorbents can be basically classified into the following categories: bacteria (e.g. Bacillus subtillis), fungi (e.g. Rhizopus arrhizus) (Table 3), yeast (e.g., Saccharomyces cerevisiae), algae, industrial wastes (e.g., S. cerevisiae waste biomass from fermentation and food industry), water plants (e.g. Water Hyacinths and Reeds), agricultural wastes (e.g. corn core), and other polysaccharide materials [9].
The importance of metallic ions to fungal and yeast metabolism has been known for a long time [10]. The yeast biomass has been successfully used as biosorbent for removal of Ag, Au, Cd, Co, Cr, Cu, Ni, Pb, U, Th and Zn from aqueous solution. Yeasts of genera Saccharomyces, Candida, Pichia are efficient biosorbents for heavy metal ions [11].
Algae are of special interest in search for and the development of new biosorbents materials due to their high

Table 1. Biomass and their biosorbent capacity [12].

Table 2. Metal biosorption capacity by different biosorbents.

Table 3. The value of sorption capacity (mmol/g) for different sorbents [23].
sorption capacity and their ready availability in practically unlimited quantities in the aquatic systems as seas and oceans (Table 4) [24,25].
Aquatic systems are considered as natural ecosystems that are designed to take advantage of the natural processes to provide efficient and low-cost wastewater treatment. The removal of metals from the water column within an aquatic system is performed generally by biological species uptake and by adsorption to sediments. Therefore, the aquatic systems such as streams, rivers, reservoirs and lakes serve as sink for heavy metal in aqueous solutions. The metal ion bioavailability for sorption to the biotic surface is pH dependent, as well for metal ion adsorption to sediments. The binding of a metal ion to the biotic surface of an organism decreases with increasing pH, whereas the binding behavior of metal ion to sediments increases with increasing pH.
2. Methodology
The research methodology implies Neuro-Fuzzy system to model and assess mercury removal from aquatic natural systems. Investigational data and information for mercury

Table 4. Algae sorption capacity (mmol/g) for metal ions in aqueous solution.
bioavailability in water and adsorption to sediments were adopted from previous research work-literature review of Prof. Elektorowicz research team attained in Concordia University-Canada [26-32].
Neuro-Fuzzy system simulates mercury sorption and evaluates the efficiency of removal by verifying the effects of pH and mercury concentration in water. The computational tools used in this research are those in MATLAB; fuzzy toolbox and simulink.
3. Adaptive Neuro-Fuzzy Inference System (ANFIS)
In natural systems where variables are interrelated and data is large, it is difficult to determine the membership functions for input variables. Neuro-adaptive learning technique works similarly to that of neural networks. It provides a method for the fuzzy modeling procedure to learn information about a data set. Fuzzy Logic computes the membership function parameters that best allow the associated fuzzy inference system to track the given input/output data. The Fuzzy Logic accomplishes this membership function parameter adjustment is called adaptive neuro-fuzzy inference system. Using a given input/ output data set, the fuzzy inference system uses either a backpropagation algorithm alone or in combination with a least squares type of method. This adjustment allows fuzzy systems to learn from the data they are modeling. Then testing the data to check the generalization capability of the resulting fuzzy inference system is needed.
Checking the data is set for model validation. Model validation is the process by which the non-trained input variables are presented to the trained fuzzy inference system model to see how well the model predicts the corresponding output data.
3.1. Adaptive Neuro-Fuzzy Inference System for Mercury Speciation
The fuzzy inference system consists of two components: the linguistic term base (database) and the rule base. The database is fuzzified in two parts: fuzzy premises (input) and fuzzy conclusions (output). The fuzzy production rule base infers input to output and then defuzzified.
Figure 1 shows a scheme for fuzzy inference system.
Adaptive Neuro-Fuzzy Inference System (ANFIS) is applied to estimate mercury removal in natural waters. ANFIS simulates and predicts mercury bioavailability that will be bio-sorbed by biological species. ANFIS model entails the following input variables to estimate output variable (bioavailable mercury concentration):
- Initial concentration of total mercury is in the range 0.3 ´ 10–6 - 1 ´ 10–3 moles/l;
- The pH value is situated in the range 5.36 - 8.
ANFIS model is constructed into two inputs (Hgi and pH), one output (Bioavailable Hg), and nine rules. ANFIS model, training the data, and training error are illustrated in Figure 2.
ANFIS model fits the experimental data for bioavailable mercury concentration. Subsequently, comparison is conducted between results obtained from the model using ANFIS and experimental results for different initial total mercury concentrations in water and pH. The comparison shows strong correlation (Figures 3 and 4).


Figure 2. ANFIS model and training for mercury bioavailability in water by varying initial Hg concentration and pH.

Figure 3. Simulation of bioavailable mercury concentration to be uptaken by bio-species verses pH for the range of initial mercury concentration.

Figure 4. Simulation of bioavailable mercury concentration to be uptaken by bio-species versus initial total mercury concentrations and pH of 5.36.
3.2. Adaptive Neuro-Fuzzy System for Mercury Adsorption
In the second stage of work, ANFIS provides solution for soil adsorption of mercury for different conditions of initial mercury concentration and pH value. Neuro-Fuzzy system depends on fuzzy knowledge bases that satisfy the following parameters:
- The initial concentration of total mercury is in the range between 1 ´ 10–7 to 1 ´ 10–3 mole/l;
- The pH value is located between 5.36 to 8;
- The adsorbent concentration is 10 g/l.
ANFIS model is consisted of two inputs (Hgi and pH), one output (adsorbed Hg), and nine rules. The model and training the data are shown in Figure 5. ANFIS model shows strong correlation between simulation and experimental data of mercury adsorption within different initial mercury concentrations and pH as shown in Figures 6 and 7.
For certain initial mercury concentrations, the adsorbed mercury is decreasing from its upper value when pH equals 8 to its lower value and when pH equals 5.36 as shown in Figure 6. The benefit of neural training of the fuzzy inference system is vital especially when there is large data and no experience of the system behavior.
4. Simulation and Forecasting
In previous sections the ANFIS model is constructed, trained, and checked. Now the model is ready for further range of simulation and forecasting. More information could be predicted for mercury removal by sorption in aquatic natural systems. In this section a simulink diagram is used for forecasting. Figure 8 shows an example of using fuzzy logic systems for mercury bioavailability and adsorption that were produced in previous sections to expand more information about Hg removal.
The run of simulink model describes the total mercury removal performance by components of an aquatic natural system; this performance can be depicted within different pH at certain initial mercury concentration. Figure 9 shows the performance of an aquatic system where it is optimal at pH equals 6.5. The fitting equation in the figure provides forecasting for total removal of mercury by natural waters components (bio-species and sediments) at any value of pH. The analysis in this
 (a)
(a)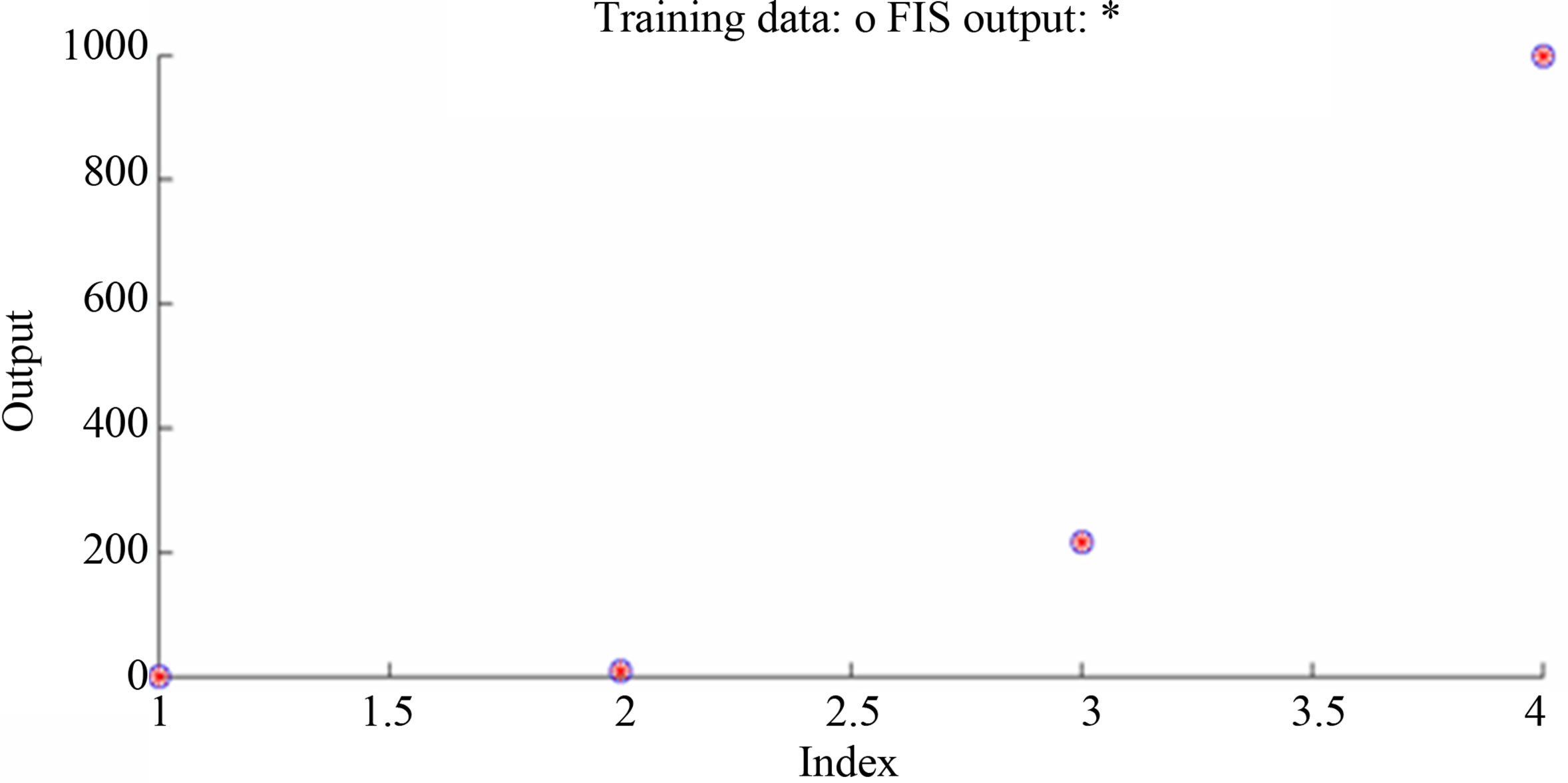 (b)
(b)
Figure 5. (a) ANFIS model and (b) training data: for mercury adsorption to sediments by varying initial Hg concentration and pH.

Figure 6. Comparison between ANFIS simulation and experimental data for adsorbed Hg within different initial Hg concentration, when pH is varying as 8, 6.5, and 5.36 respectively at each concentration.

Figure 7. Comparison between ANFIS simulation and experimental data for Adsorbed Hg concentration within variable pH in Solution.

Figure 8. Simulink diagram forecast for total mercury removal for different Hgi and pH variables.

Figure 9. Total mercury removed by natural water within different pH, and the forecast equation.
section supply more information for model performance and forecasting. It also gives information about removal efficiency of the overall system.
5. Conclusion
Fuzzy logic proved to be useful for assessing ambiguous natural processes. Modeling of mercury bioavailability for bio-species and adsorption to sediments shows strong correlation of more than 98% between simulation results and experimental data. Using adaptive neuro-fuzzy system is important for hazy system and no experience about data behavior. The findings of this research provide information, simulation, and forecasting about heavy metal removal efficiency in natural systems.
6. Acknowledgements
The financial support from the Natural Sciences and Engineering Research Council of Canada under grant RGPIN-18948 is gratefully acknowledged.