Groundwater Quality Analysis of Safidon and Julana Blocks of District Jind, Haryana, India ()
1. Introduction
Water quality is an important factor in determining the human welfare. Water quality means the description of the chemical, physical and biological characteristics of water. Water is essential to life and for the harmonious development of our bodies, as it is involved in a number of biological processes. Water is used for drinking, irrigation, sanitation and many other human needs. It affects our lifestyle and economic well-being. More than three quarters of the earth’s surface is made up of water and only 2.8 percent of the earth’s water is available for human consumption and the rest 97.2 percent is in the oceans. Rapidly increasing population, rising standards of living and exponential growth of industrialization and urbanization have exposed the water resources and has degraded the water quality. The major sources of water pollution are domestic waste and industrial wastes, which are discharged into natural water bodies [1-3]. Pollutants such as herbicides, pesticides, fertilizers, hazardous polychlorinated biphenyls and synthetic organic chemicals can also make their way into water supply. Polluted ground water is a major cause for the epidemic and chronic diseases in human beings. Many investigations have found a correlation between cardiovascular deaths and water composition [4,5]. The disorder of teeth and bones is due to consumption of fluoride-rich water [6]. Carbonate and bicarbonate may originate from microbial decomposition of organic matter also. Alkalinity is big problem for industries also, as if alkaline water is used in boilers for steam generation, it may lead to precipitation of sludge, deposition of scales and causes caustic embrittlement [7]. The residential waste is discharged into the pits, ponds due to which the waste migrates down to the water table [8] and moreover there is a possibility of dissolution of rocky materials in the area.
Due to less availability and non-acceptance of surface water, people of Haryana have to depend upon groundwater resources to a large extent. In many areas of the state, ground water is the only source for drinking water. The main sources of drinking water in rural areas are fresh water body such as wells, tube-wells and hand pumps. In urban areas the municipal supply water is available only for short periods and for limited time while in rural areas the supply water is even after 4 - 5 days. Therefore, people are compelled to use ground water for drinking and cooking purposes both in rural and urban areas. The groundwater is used for the irrigation purpose as well and the irrigation schemes play a vital role in the economy of any region, state or country [9]. The problem of higher fluoride concentration, high TDS, high cation and anion concentration in groundwater has now become one of the most important toxicological and geo-environment issues in India. Recently, various groups [10-12] have analysed groundwater samples for their physiochemical parameters.
The Jind district is in the North of Haryana state of India between 29.03' and 29.51' North latitude and 75.53' and 76.47' East longitude. Panipat, Karnal and Kaithal districts, respectively, are on its East and North-East. In the West and South-West it has a common boundary with district Hisar and Fatehabad and in its South and SouthEast lie the district of Rohtak and Sonipat, respectively. Its boundary line on the North forms the inter-state Haryana-Punjab border with Patiala and Sangurar districts of Punjab. Safidon and Julana are tehsils in District Jind of Haryana state of India. Safidon is the centre of Panipat and Jind districts. It is situated on the bank of the Hansi branch of the Western Yamuna canal, 35 km northeast of Jind. Julana is located in the middle of the Rohtak and Jind districts. Entire drinking water supply to all rural as well as urban parts of the Julana and Safidon blocks of Jind district is based on groundwater from hand pumps, tube wells or by canals. The objective of this study is to present the quality of drinking water supply sources in some of the locations of Safidon and Julana blocks in District Jind, Haryana, India.
2. Sampling and Physio-Chemical Methods of Analysis
Groundwater samples from thirty six locations of Safidon block and thirty five locations of Julana block in District Jind, Haryana, India, were analyzed for their physiochemical parameters. Water samples were collected from different localities in the cleaned polythene bottles. The bottles were well rinsed before sampling and tightly sealed after collection and labelled in the field. Sampling was carried out without adding any preservative. The sampling locations are given in Table 1. The physio-chemical analysis of water samples was carried out for various quality parameters such as pH, electrical conductivity (EC), total dissolved solids (TDS), total alkalinity (TA), total

Table 1. Sampling locations of Safidon block and Julana block in District Jind, Haryana, India.
hardness (TH), sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), carbonate  , bicarbonate
, bicarbonate  , chloride (Cl–), sulphate
, chloride (Cl–), sulphate 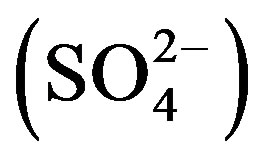 and fluoride (F–) as per the standard procedure described by the “Standard Methods For The Examination of Water and Wastewater American Public Health Association (APHA)” [13]. The pH, EC, TDS and salinity of all the water samples were determined using digital portable kit (Electronics India, Panchkula, India). Calcium (Ca2+), Magnesium (Mg2+) and total hardness (TH) were determined by the Ethylene Diamine Tetra Acetic Acid (EDTA) titration method, Chloride (Cl–) by Argentometric titration method. The total alkalinity (TA) was determined using the titration method. Fluoride (F–) was determined using Alizarin spectrophotometer.
and fluoride (F–) as per the standard procedure described by the “Standard Methods For The Examination of Water and Wastewater American Public Health Association (APHA)” [13]. The pH, EC, TDS and salinity of all the water samples were determined using digital portable kit (Electronics India, Panchkula, India). Calcium (Ca2+), Magnesium (Mg2+) and total hardness (TH) were determined by the Ethylene Diamine Tetra Acetic Acid (EDTA) titration method, Chloride (Cl–) by Argentometric titration method. The total alkalinity (TA) was determined using the titration method. Fluoride (F–) was determined using Alizarin spectrophotometer.
3. Results and Discussion
Characterizations of the physio-chemical parameters of different locations in Safidon block and Julana block of District Jind, Haryana, India are reported in Tables 2 and 3, respectively. The experimental results of the Safidon block and Julana block are compared in Tables 4 and 5, respectively, with the standard limits recommended by the World Health Organization (WHO) [14] and ISI [15]. Considerable deviations are observed in the water quality parameters from the standard limits.
3.1. pH
The permissible limits for drinking water are 7.0 - 8.5. In absence of any alternate source, water with pH 6.5 - 9.2 can be used. The pH values of the samples in Safidon block ranges from Safidon 7.2 to 9.3 with average 8.54. The pH values of the samples in Julana block ranges from 7.16 to 8.73 with average 7.84. The ground waters are slightly alkaline in nature.
3.2. Electrical Conductivity
EC of the samples from the Safidon block ranges from 1.1 mS to 5.7 mS with a mean of 2.05 mS and those of the samples from Julana block ranges from 1.12 mS to 8.07 mS with a mean of 3.46 mS.
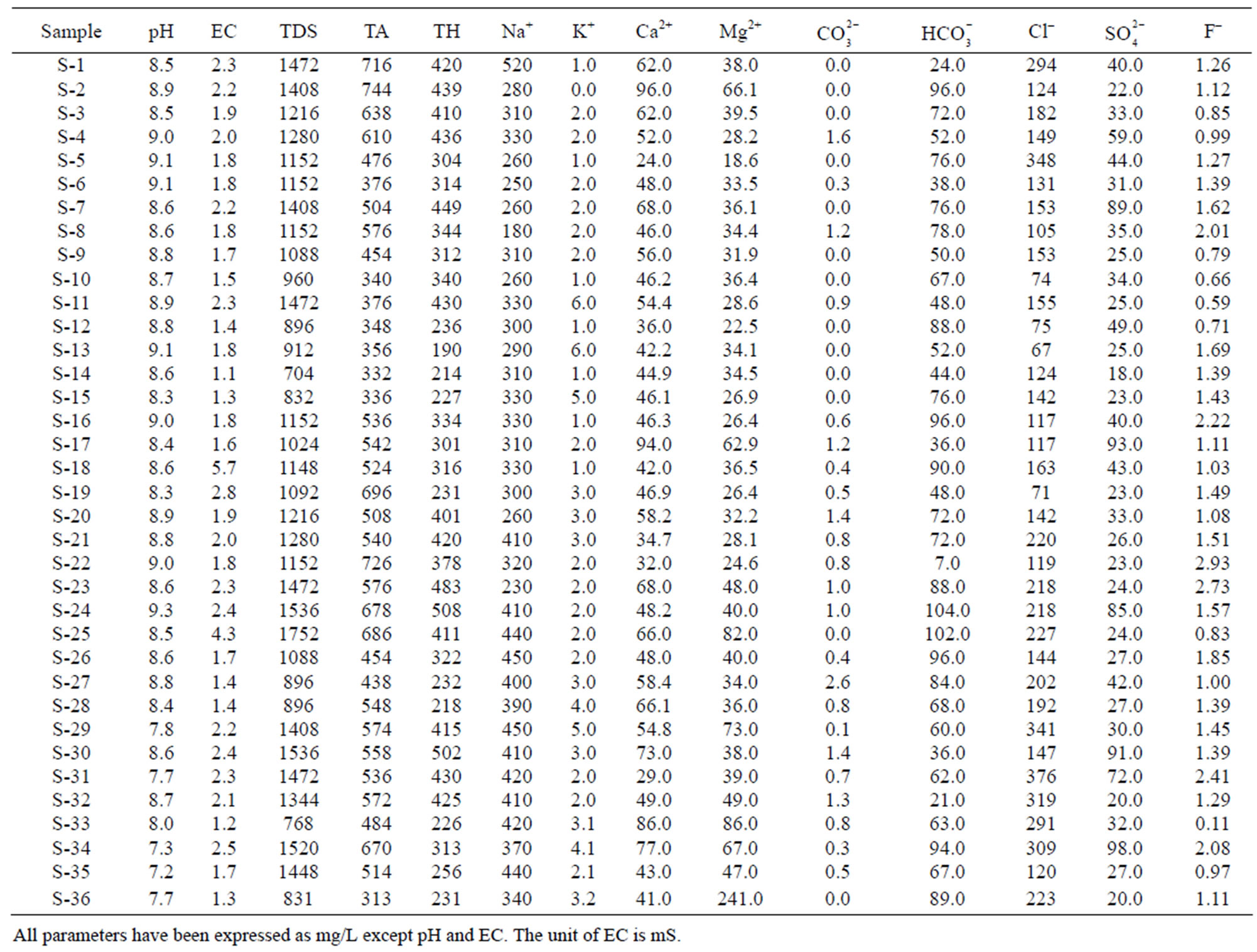
Table 2. Physico-chemical characteristics of ground water of Safidon block.
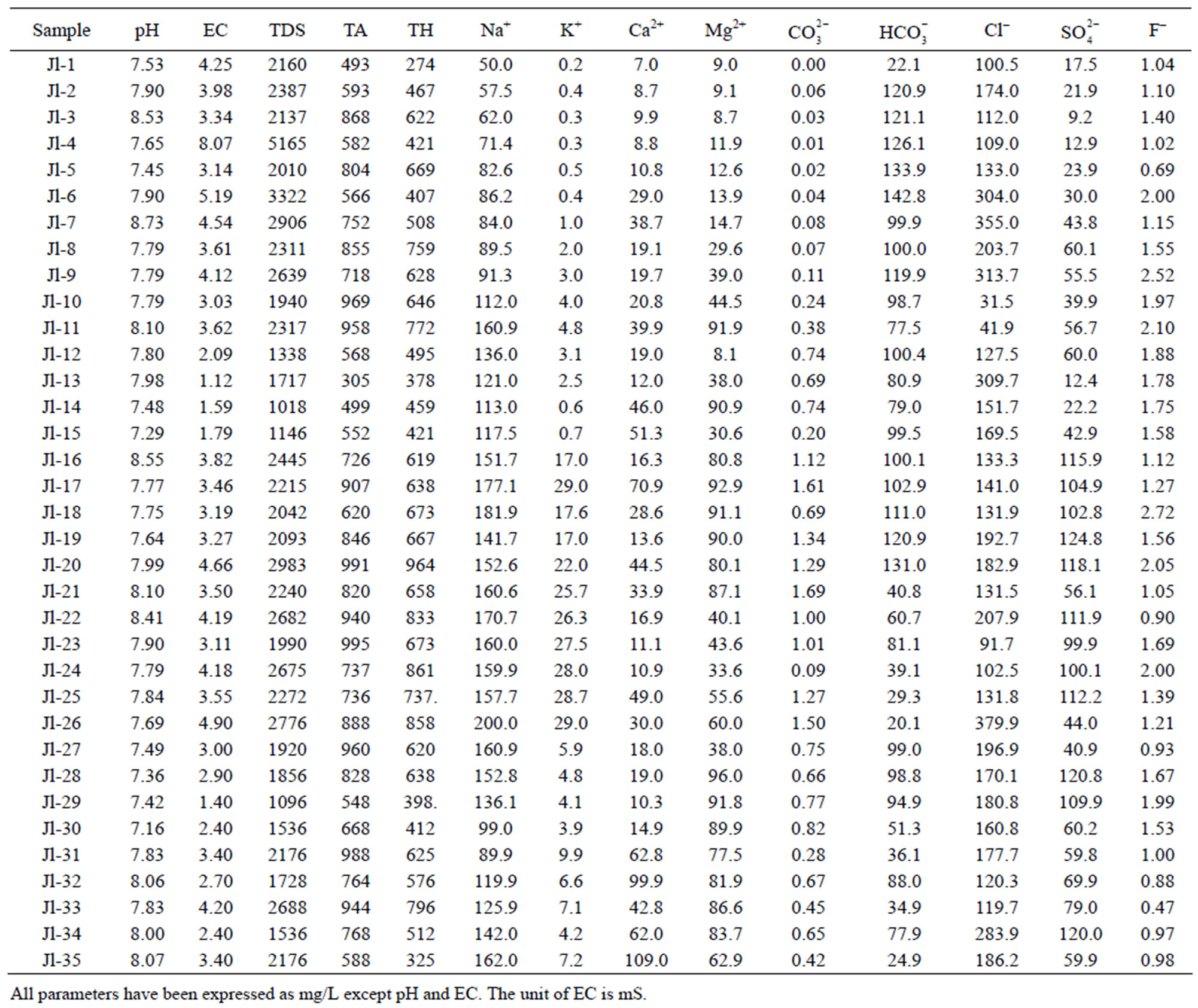
Table 3. Physiochemical characteristics of ground water of Julana block.

Table 4. Comparison of groundwater of Safidon block with drinking water standards (Indian and WHO).

Table 5. Comparison of groundwater of Julana block with drinking water standards (Indian and WHO).
3.3. Total Dissolved Solids
According to WHO, the maximum acceptable concentration of TDS in groundwater for domestic purposes is 500 mg/L and excessive permissible limit is 1500 mg/L. TDS values of the samples from Safidon block ranges from 704 mg/L to 1752 mg/L with mean value 1198.2 mg/L. TDS values of the samples from Julana block ranges from 1018 mg/L to 5165 mg/L with a mean of 2218.2 mg/L. According to classification of drinking water on the basis of TDS values, water of 25% samples from Safidon block was found to be non-saline and water of 75% samples was found to be slightly saline. No any sample of water was found to very saline. TDS values of all samples of Julana block were greater than acceptable WHO standards (500 mg/L). Classification of drinking water of Julana and Safidon blocks of Jind district on the basis of TDS values is given in Table 6.
3.4. Total Hardness, Calcium and Magnesium
Total hardness is an important parameter of water for its use in domestic sector. Calcium and magnesium are important parameter for total hardness. The acceptable limits of Ca2+ and Mg2+ in water for domestic use are 75 and 200 mg/L respectively. In case of non-availability of alternate source of water, Ca2+ and Mg2+ limit can be extended upto 200 and 400 mg/L. In the water samples of Safidon block, the total hardness ranges from 190mg/L to 508 mg/L with a mean value 344.9 mg/L. The Ca2+ concentration ranges from 24 mg/L to 96 mg/L with a mean value of 54 mg/L. The Mg2+ value are in between 18.6 mg/L to 241 mg/L with a mean value of 46.3 mg/L. In the groundwater of Julana block, the total hardness of water samples ranges from 274 mg/L to 964 mg/L with a mean of 600.25 mg/L. Ca2+ concentration in water samples from all the locations was found to vary from 7 mg/L to 109 mg/L. Mg2+ concentration in water samples from all the locations ranged from 8.1 mg/L to 92.9 mg/L. Classification of drinking water of Julana and Safidon blocks of Jind district on the basis of TH values is given in Table 7.
3.5. Total Alkalinity, Carbonate and Bicarbonate
The acceptable limit of total alkalinity in drinking water is 200 mg/L. Beyond this limit, taste of water become unpleasant, whereas in absence of alternate water source, alkalinity upto 600 mg/L is acceptable. The values of alkalinity in the water samples of Safidon block are in between 313 mg/L to 744 mg/L. The concentration ranges from 0 mg/L to 2.6 mg/L. The concentration is between 7 mg/L to 104 mg/L. In the water samples of Julana block, the TA ranges between 305 mg/L to 995 mg/L. The average total alkalinity was 752.7 mg/L. The concentration is between 0 mg/L to 6.9 mg/L. The concentration ranges from 20.1 mg/L to 142.8 mg/L.

Table 6.Classification of drinking water of Julana and Safidon blocks of Jind district on the basis of TDS values.