American Journal of Plant Sciences
Vol.4 No.8(2013), Article ID:35457,4 pages DOI:10.4236/ajps.2013.48193
Potential Breeding for High Nitrogen Fixation in Pisum sativum L.: Germplasm Phenotypic Characterization and Genetic Investigation
![]()
1Department of Crop and Soil Sciences, Washington State University, Pullman, USA; 2Department of Horticulture, Washington State University, Pullman, USA; 3ProGene Plant Research, Othello, USA.
Email: rita_ag@wsu.edu, eliane.bodah@email.wsu.edu, mike@progenellc.com, kurt@progenellc.com
Copyright © 2013 R. Abi-Ghanem et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 15th, 2013; revised June 17th, 2013; accepted July 15th, 2013
Keywords: Nitrogen Fixation; Field Peas; Agricultural Inputs
ABSTRACT
Nitrogen (N) is the most yield-limiting crop nutrient worldwide. Industrially produced N has increased in cost over the past years, and is unavailable in many regions around the globe. Biological N fixation by rhizobial bacteria is a great underutilized resource that this project aims to maximize. Grain legumes fix approximately 20 to 100 kg·N·ha−1·yr−1. The amount of N supplied by fixation is affected by genes and traits of both the bacterial and plant partners. The objectives of this study are to identify Pisum sativum varieties with high nitrogen fixation efficiency. This is achieved by germplasm screening and phenotypic evaluation of nodule formation, total plant nitrogen, and residual nitrogen in soil. Significant differences in plant total nitrogen among the various cultivated genotypes were found, with heritability of 0.57. These pea varieties left in the soil a residual N that varies between 11.21 to 65.018 kg·N·ha−1. Our findings reveal a unique opportunity for improving N fixation through genetic crossing and selection.
1. Introduction
Symbiotic legume-rhizobial bacteria interactions have been estimated to contribute 91 to 163 million tons of nitrogen per year, 65% of this is used in agriculture [1]. Biological nitrogen fixation efficiency depends on the type of rhizobia strain, crop variety, and interactions between specific strains and crop varieties as well as environmental factors and management practices. After photosynthesis, biological nitrogen fixation (BNF) by legumes might be considered the most fundamentally important biological process on the planet. This is a critical issue because many countries, both developing and developed, have not fully embraced or taken advantage of BNF potential and are substantially reliant upon fertilizer nitrogen to drive agricultural productivity. Grain legumes fix approximately 20 to 100 kg·N·ha−1·yr−1 and use almost the entire amount of fixed nitrogen in grain production [2]. Many USA extension publications tend to credit only 10 to 25 kg·N·ha−1 for previous grain legume crops. At least part of the cause for this variable level of fixation and low nitrogen credit from grain legumes is the fact that these legumes have been bred for yield but have not been bred for nitrogen fixation (K. McPhee, pers. comm.). In addition, inoculant companies have focused strain formulations not on the ability to fix large amounts of nitrogen, but on the ability to associate with many different legume varieties. A large genetic variation for nodule number and weight has been observed in legume crops, including peas, which are positively associated with BNF ability. Recent studies have shown that there are differences in nitrogen fixation among genotypes, for example, the “Shawnee” pea variety fixed the highest percentage of total plant nitrogen over other varieties in a greenhouse experiment [3]. Legume varieties had a significant influence on BNF, suggesting that breeding for higher rates of nitrogen fixation may be possible. Lack of reliance on BNF is attributed to many factors, which range from a paucity of knowledge and expertise in manufacturing inoculants, growing and inoculating legumes with rhizobia, to government subsidies in some advanced economies [4]. Unfortunately, with the price of fossil fuels inevitably increasing, small economies will be faced with either food shortages or high costs for fertilizer N. At the same time, declining purchasing power in many developing countries will have significant implications for food production. This problem must be addressed now because current reviews forecast that food production would need to double by 2020 to feed our expanding population [5], and this cannot happen without exogenous N inputs. Biological nitrogen fixation by rhizobial bacteria is a greatly underutilized resource that this project aims to maximize by identifying Pisum sativum varieties with high N-fixing efficiency and selecting for varieties needed in breeding for nitrogen fixation. Therefore, our main objectives are to identify Pisum sativum varieties with high nitrogen fixation efficiency by germplasm screening and phenotypic evaluation of nodule formation, total plant nitrogen, and residual nitrogen in soil.
2. Materials and Methods
Eleven Pisum sativum varieties were planted in a Randomized Complete Block Design, with nine replicates, three locations in the field, in a non-irrigated area of the Pacific North West, North of Moscow, ID, USA during the summer of 2012. Nine of those varieties belonged to spring, winter and forage types and are described here as Grain A, B and C (grain type peas), Pro A, B and C (lines from ProGene Plant Research), For A and B (forage types) and Win A (winter variety). Two varieties were controls for low nitrogen fixation, the non-nodulating mutant PI 598370 conditioned by a monogenic recessive allele that was acquired from the Germplasm Resources Information Network [6], and the variety “Lifter” [7] that was reported as low performance in a greenhouse experiment conducted by Abi-Ghanem et al. [3]. Plant material was not inoculated with any Rhizobium sp., relying only on the strains present in the soil. Site selection was made observing the following criteria: history of legumes in the site, peas are rotation crops and the previous crop was winter wheat, low nitrogen (less than 200 kg·ha−1), and site homogeneity. For site homogeneity, an area with mild slope was chosen and nutrient soil profile samples were collected. Data was collected for both plant and soil analysis at four time points: prior to planting (observing the seed content), emergence, flowering, and physiological maturity. Soil Nutrients (Mo, P, Fe, Ca, S, Co, Ni, Mg, Al and Mn) in addition to exchangeable ammonium and nitrate were measured along with soil acidity, salinity and temperature. For plant analysis, samples were extracted from the ground and roots were washed in distilled water. The number of nodules and plant fresh weight were measured, and then the whole plant was dried, weighed again, ground, and sent for plant total nitrogen analysis at Kuo Testing Lab Inc. (Othello, WA, USA). Total N was determined by LECO’s Nitrogen (or Protein) Analyzer Model FP-528 with auto sampler (St. Joseph, MI). The nitrogenous compounds in the sample were combusted to nitrogen gas and then analyzed via infrared detector. Both soil available nitrateN and ammonium-N were determined by O-I Analytical Flow Injection Analyzer (FIA) (College Station, TX) with auto sampler after soil samples were extracted with 1 N KCl solution. The EPA’s cadmium reduction method was used for nitrate-N determination. The ammoniumnitrogen was determined with sodium nitroferricyanide and Clorox solution. Both color complexes of nitrate-N and ammonium-N were analyzed by on-line colorimeters. In order to help answer practical questions of how much nitrogen residue is left in the field, another sample was taken at harvest, in which the below and above ground plant mass was separated, weighed, dried, weighed again and sent to total nitrogen analysis to compare with previous time points and soil analysis. Data statistical analysis was performed using using Minitab 16 Statistical Software and Agrobase Generation II® Agronomix Software.
3. Results and Discussion
Among the results (with α = 0.05) it was observed that nodule dry weight, plant weight, total nitrogen, soil NO3 and NH4 changed significantly over time (p-value = 0.00), having its peak during flowering (p-value = 0.012). No significant difference on nodule dry weight among varieties was found, even though the non-nodulating mutant did not have any nodules. In addition, no significant differences in the varietal weight gain pattern during development were found. Significant differences were found in plant total nitrogen, with the non-nodulating mutant being the lowest and following a different pattern during development than the other varieties (Figure 1). The heritability was calculated to be 0.57, which can bring some hope for plant breeders since more than half of this trait (total nitrogen) was due to genetic control in our experiment. Plant total nitrogen also has a correlation of 0.97 with plant dry weight. However, nodule dry weight has not shown significant correlation with nitrogen fixation. This fact can be explained by a coefficient of variance over 15%.
Regarding soil analysis, it was observed that the Least Significant Difference (LSD) data for both soil NH4 as well as for NO3, separated the non-nodulating mutant into its own group, with the lowest content of soil NO3- N while For B had the highest content of soil NO3-N (Figure 2) at the last sampling time. Besides the variation encountered during development, it was possible to observe that overall there was an increase of soil NO3-N during the last sampling time. It confirms the results of a previous study conducted by Wall [8,9] that found a
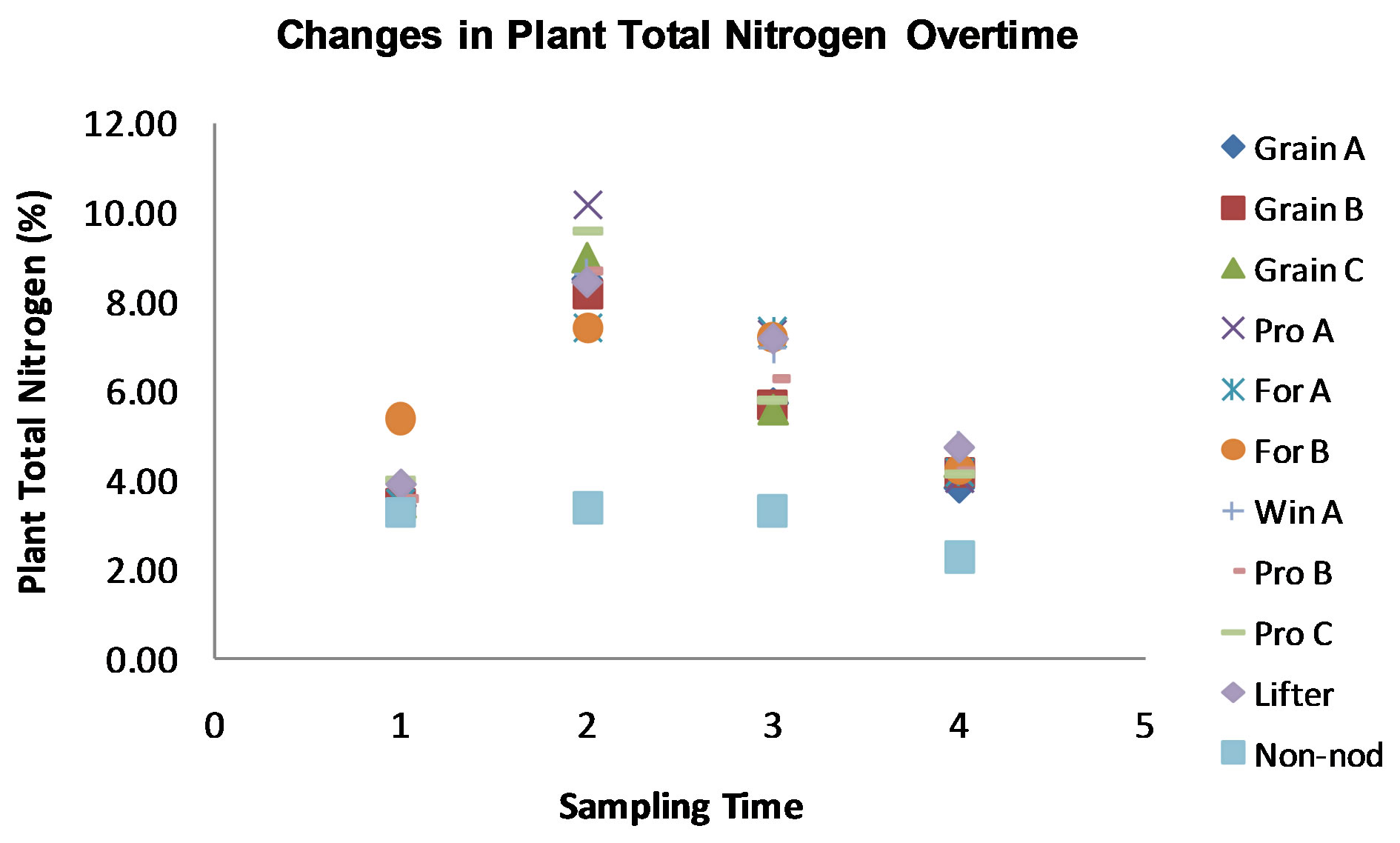
Figure 1. Changes in plant total nitrogen overtime in four time points: prior to planting (observing the seed content), emergence, flowering, and physiological maturity. A peak on plant total nitrogen was observed in the second sampling time. The legend abbreviations are as follows: Grain A, B, and C correspond to grain type peas. Pro A, B and C are lines from ProGene Plant Research. For A and B are forage type peas. Win A is a winter pea. Lifter is an USDA variety, and the Non-nod is the non-nodulating mutant PI 598370.

Figure 2. Changes in Soil NO3-N Overtime. The non-nodulating mutant had the lowest content of soil NO3-N while For. B had the highest content of soil NO3-N at the last sampling time. The legend abbreviations are as follows: Grain A, B and C correspond to grain type peas. Pro A, B and C are lines from ProGene Plant Research. For A and B are forage type peas. Win A is a winter pea. Lifter is an USDA variety, and the Non-nod is the non-nodulating mutant PI 598370.
small increase in soil just after soybean harvest and a significant increase after subsequent months.
As mentioned previously, to help answer practical questions of how much nitrogen residue is left in the field, plant above and below ground were collected at harvest. Then, total nitrogen analysis was conducted and compared with previous time points as well as soil analysis. It was observed that one variety, Grain B, had even lower nitrogen content than the non-nodulating mutant while For A had the highest content below ground (Figure 3). Moreover, forage as well as winter varieties tended to have higher total nitrogen content. The variety Lifter
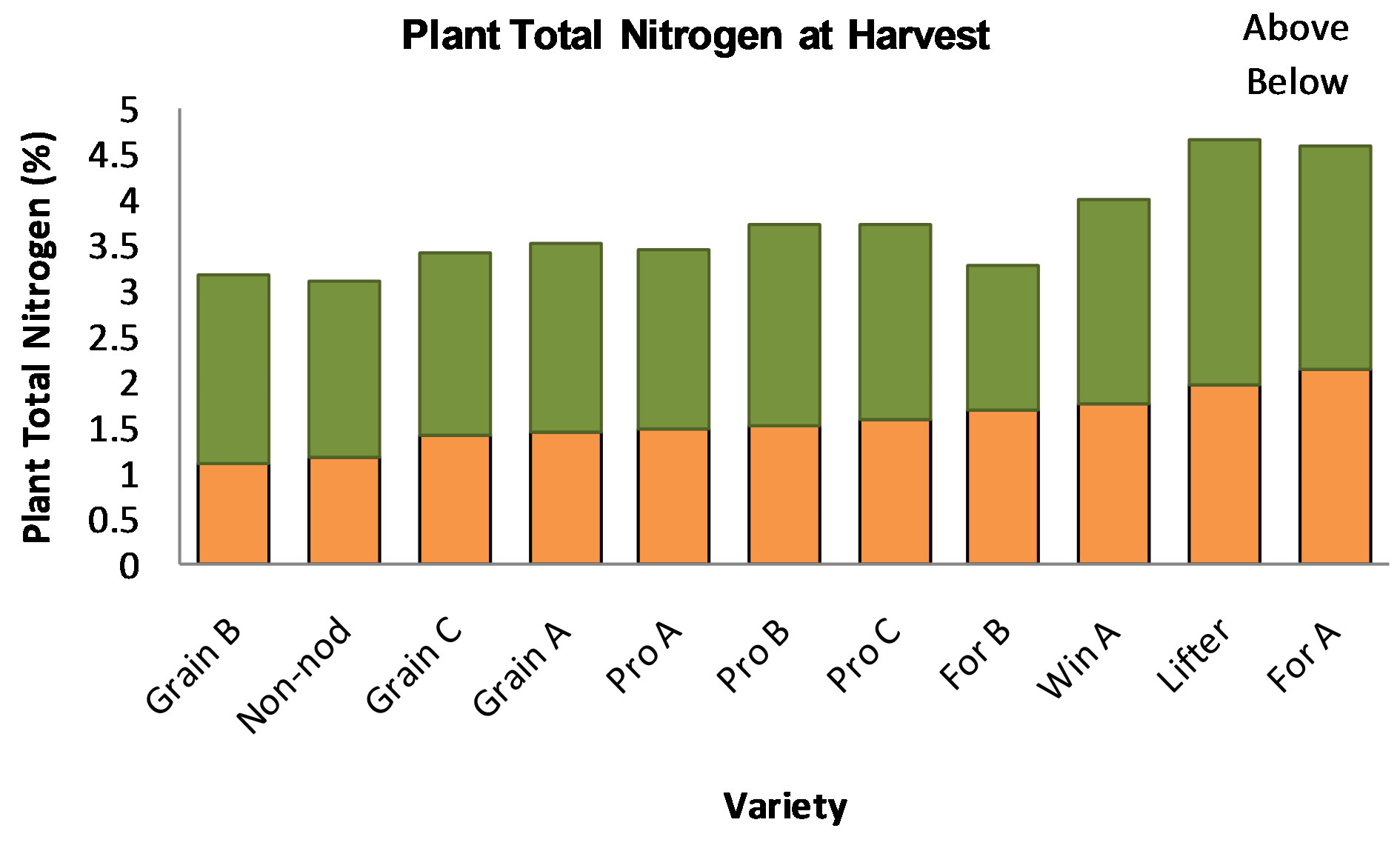
Figure 3. Plant total nitrogen below and above ground measured at harvest. Grain B, had even lower nitrogen content than the non-nodulating mutant while For A had the highest content below ground. The legend abbreviations are as follows: Grain A, B and C correspond to grain type peas. Pro A, B and C are lines from ProGene Plant Research. For A and B are forage type peas. Win A is a winter pea. Lifter is an USDA variety, and the Non-nod is the nonnodulating mutant PI 598370.
performed better in our field experiment than the greenhouse experiment conducted by Abi-Ghanem et al. [3], which suggests that field experiments have different results and should be conducted for breeding and variety selection.
A study conducted in Montana observed that a harvested grain legume can supply the next crop up to 22.4 kg·N·ha−1 [10]. Another study, on lentils, concluded that estimates of the percentages of plant nitrogen derived from fixation ranged from 28% to 87% with an average of 63% [11]. In our study, when converting NO3-N + NH4-N ppm to kg·N·ha−1, it is noteworthy to mention that our varieties of Pisum left in the soil 11.21 to 65.018 kg·N·ha−1 of residual nitrogen. As pulses are used in rotations, the majority of plant growers sow a second crop fairly soon after harvest and this analysis can contribute to the amount of artificial fertilizer needed in the field, lowering inputs and agricultural costs. Despite the fact that there were no significant differences found among market classes (spring, winter and forage types), significant differences in plant total nitrogen among genotypes were found, with heritability of 0.57. This reveals an opportunity for improving nitrogen fixation ability through genetic crossing and selection, which leads to conclude that breeding efforts should be applied. Breeding for high nitrogen fixation also involves many phenotypic traits, for example, Sidorova [12] has shown that broad expansion of the pea root system is possible through breeding.
4. Conclusion
Varietal significant differences were found for plant total nitrogen in this study and it was possible to observe that breeding for high nitrogen fixation is a promising area of research. The amount of nitrogen supplied by fixation is affected by genes and traits of both the bacterial and plant partners besides abiotic and edaphic conditions, as well as interactions with other microorganisms. The host plant produces lectins, nodulin protein, leghemoglobin, and provides carbohydrates to the bacterial symbiont. The legume-rhizobial symbiotic proccess involves multiple steps and genes but it provides opportunities to breed legume crops with layered or pyramidal host abilities. Most essential genes for nitrogen fixation are known, such as genes for nodulin, lectins, and malate dehydrogenase, but crops have not been developed to optimize their expression. Quantitative trait locus mapping is needed for multi-component factors including nodule number on inoculated plants, nodule mass, total nitrogen fixation, and nitrogen fixation efficiency. Overall, this study was important to observe nitrogen fixation in the field with native bacterial strains and to identify potential parental germplasm with significant differences in nitrogen fixation. High throughput genotyping as well as monitoring symbiotic interactions must be evaluated to promote a better understanding of the breeding steps for high nitrogen fixation. In addition, more phenotypic studies increasing the number of tested varieties are necessary in order to evaluate related traits.
5. Acknowledgements
Chris Braunwart, Patrick Dailey, Paula and Jacob Smith —ProGene Plant Research; Brian Bodah—Washington State University; Crites Seed Inc. and Plant Research LTD-NZ.
REFERENCES
- R. H. Burris and G. P. Roberts, “Biological Nitrogen Fixation,” Annual Review of Nutrition, Vol. 13, 1993, pp. 317-335. doi:10.1146/annurev.nu.13.070193.001533
- J. Evans, G. E. O’Connor, G. L. Turner, D. R. Coventry, N. Fettell, J. Mahoney, E. L. Armstrong and D. N. Walsgott, “N2 Fixation and Its Value to Soil N Increase in Lupin, Field Pea and Other Legumes in South-Eastern Australia,” Australian Journal of Agricultural Research, Vol. 40, No. 4, 1989, pp. 791-805. doi:10.1071/AR9890791
- R. Abi-Ghanem, L. Carpenter-Boggs and J. L. Smith, “Cultivar Effects on Nitrogen Fixation in Peas and Lentils,” Biology and Fertility of Soils, Vol. 47, No. 1, 2011, pp. 115-120. doi:10.1007/s00374-010-0492-6
- J. G. Howieson, R. J. Yates, K. J. Foster, D. Real and R. B. Besier, “Prospects for the Future Use of Legumes,” In: M. J. Dilworth, E. K. James, J. I. Sprent and W. E. Newton, Eds., Nitrogen-Fixing Leguminous Symbioses, Springer, Amsterdam, 2008. doi:10.1007/978-94-011-4385-1_2
- D. Byerlee and R. White, “Agricultural Intensification and Diversification through Food Legumes: Technological and Policy Options,” In: R. Knight, Ed., Linking Research and Marketing Opportunities for Pulses in the 21st Century, Kluwer Academic Publishers, Dordrecht, 2000.
- GRIN—Germplasm Resources Information Network, 2012. http://www.ars-grin.gov
- United States Department of Agriculture, “Notice of Release of Lifter Dry Pea,” 2012. http://washingtoncrop.com/documents/Field%20Peas/Lifter.pdf
- D. P. Wall, “Soil Tests for Winter Wheat Nitrogen Management in the Southeastern USA,” UMI Dissertation Publishing, 2009.
- T. Hu and J. P. Desai, “Soft-Tissue Material Properties under Large Deformation: Strain Rate Effect,” Proceedings of the 26th Annual International Conference of the IEEE EMBS, San Francisco, 1-5 September 2004, pp. 2758-2761.
- K. Olson-Rutz, C. Jones and P. Miller, “Soil Nutrient Management on Organic Grain Farms in Montana,” MSU Extension Service, Montana State University, Bozeman, 2010.
- M. A. Quinn, “Biological Fixation and Soil Health Improvement,” In: W. Erksine, F. J. Muehlbauer, A. Sarkar and B. Sharma, Eds., The Lentil: Botany, Production, and Uses, CABI, Wallingford, 2009, pp. 220-247. doi:10.1079/9781845934873.0229
- K. K. Sidorova, “Use of Supernodulating Mutants in Pea Breeding,” Pisum Genetics, Vol. 43, 2011.

