Open Journal of Obstetrics and Gynecology
Vol. 2 No. 1 (2012) , Article ID: 18351 , 8 pages DOI:10.4236/ojog.2012.21008
Influence of obstetric factors on the development of postpartum antithyroid antibodies
![]()
Clínica Morales, Valencia, Spain
Email: cm@comv.es
Received 30 December 2011; revised 30 January 2012; accepted 25 February 2012
Keywords: Antithyroid antibodies; maternal screening; microchimerism; Postpartum thyroiditis
ABSTRACT
Aim: To correlate obstetric data with the appearance of antithyroid antibodies. Methods: A 6 months follow up was performed on 135 healthy women with assessment of TSH, T3, T4 and antithyroid antibodies (anti thyroglobulin and anti peroxidase). Correlation of diverse obstetrical parameters with the appearance of antithyroid antibodies at 2 and 6 months postpartum was determined. Results: Only two parameters were significant during the complete follow up: the newborn weight, which correlated with both antibodies (anti-thyroglobulin and anti-peroxidase) positivity and the maternal height, which exclusively correlated with anti-thyroglobulin positivity. Conclusions: Correlation of maternal height and newborn weight with positive autoantibodies allows to consider a future clinical screening test for this disorder.
1. INTRODUCTION
Postpartum thyroiditis is a rather frequent thyroid disfunction affecting women during puerperium as a consequence of an autoimmune inflammation of the thyroid gland. It is the most frequent thyroid disorder at this period and normally develops with a brief hyperthyroid phase followed by a hypothyroid period, which may end in a permanent hypothyroidism or a spontaneous recovery of thyroid function [1-3].
Prevalence of postpartum thyroiditis widely varies between 1.1% in Thailand to 21.1% in the USA [2-21]. Its etiology is not known, although it is related with autoimmunity through the development of antithyroid antibodies: anti-thyroglobulin (anti-Tg) and anti-peroxidase (anti-TPO).
The thyroid dysfunction produced has a major importance for female health, as most women affected are not diagnosed and many years later present overweight, goiter and other anomalies due to long term hypothyroidism such as heart disease, cracked skin, susceptibility to infection, depression, mental confusion, fatigue, osteoporosis and anomalies of the blood lipid profile.
Currently, we do not have any screening for postpartum thyroiditis and no studies correlating obstetric data with appearance of antithyroid antibodies have been performed.
In order to find useful clinical parameters to design a screening test for postpartum thyroiditis, we considered the performance of a study with a 6 months follow up, correlating several thyroid analytical parameters with a variety of obstetric data in order to evaluate if any of these behaved as a significant factor for the appearance of antithyroid antibodies.
2. MATERIALS AND METHODS
We studied a sample of 135 healthy women attending a private clinic of whom we obtained an obstetrical and neonatal complete medical history including age, blood group, number of children and miscarriages, maternal height, maternal weight at the beginning and at the end of gestation, route of delivery and data related with the newborn (weight, sex, and blood group). All patients with past medical history of thyroiditis were discarded for the study.
We subsequently did a 6 months follow up of all the patients, performing a first blood test 2 months after delivery, which included assessment of TSH, T3, T4 and antithyroid antibodies (anti-TPO y anti-Tg). This blood test was repeated 6 months later with measurement of T3, T4 and antithyroid antibodies.
For a patient to be included in the study, levels of TSH in the first measurement had to be within the normal parameters in order to rule out subclinical anomalies of thyroid function.
To simplify the statistical analysis we divided patients into positive and negative for antithyroid antibodies; Positivity of antithyroid antibodies at 2 months postpartum included patients whose antibodies production started before labor and also patients whose antibodies production was started early after labor When positivity was present only at 6 months, it included patients whose production started at any period between 2 and 6 months postpartum.
For comparative studies, the following groups were considered: 1) patients anti-Tg positive versus patients anti-Tg negative at 2 months, 2) patients anti-TPO positive versus patients anti-TPO negative at 2 months, 3) patients anti-Tg positive versus patients anti-Tg negative at 6 months and finally: 4) patients anti-TPO positive versus patients anti-TPO negative at 6 months. The characteristics of these groups were analysed and the relationship of the diverse obstetrical parameters with the appearance of antithyroid antibodies at 2 and 6 months postpartum was determined.
The following normality limits for thyroid hormones were considered: T3: 70 - 185 ng/dl, T4: 4.4 - 13 ng/dl. Antithyroid antibodies were considered normal below 50 U/ml [22]. Concerning TSH, although a controversy exists concerning its upper limit in euthyroid patients, we considered the TSH normal range between 0.1 and 5.6 mUI/l.
Physical measurements like height or weight were obtained with a Seca 220 scales. (Seca Weighing and measuring systems®. Hamburg. Germany). Fetal wellbeing control during pregnancy was done using a Toshiba Doppler color SSH 140A ultrasound machine. (Toshiba Corporation®, Japan) and a Toitu fetal monitor (Toitu CO LTD® (Tokio, Japan).
Finally, assessment of statistical significance was done with the software Statplus® version 2009 for Apple Macintosh, using the test of t-Student for numerical data and the test of Chi-square for frequency data.
3. RESULTS
Results are described in Tables 1-4.
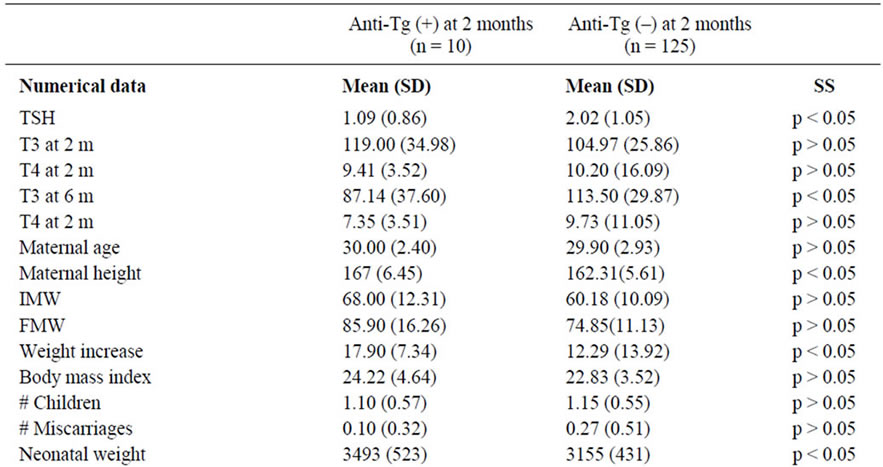

Table 1. Comparison of patients anti-Tg (+) with patients anti-Tg (–) at 2 months of puerperium.
At 2 months 10 patients (7.4%) showed positivity for anti-Tg antibodies and 13 patients showed positivity for anti-TPO antibodies. 6 patients (4.4%) showed positivity for both anti-TG and anti-TPO antibodies.
This relation was increased at 6 months: 20 patients (14.8%) and 17 patients (12.5%) respectively showed positivity for anti-Tg and anti-TPO antibodies. Also, 9 patients (6.7%) showed positivity for both anti-TG and anti-TPO antibodies.
At 2 months, and for the positivity of anti-Tg antibodies: TSH levels, T3 levels at 6 months, maternal height, maternal weight increase, newborn weight and difference between maternal and neonatal Rh were significant (Table 1). At 2 months and for the positivity of anti-TPO antibodies: T4 levels at 6 months, number of children and newborn weight were also significant (Table 2).
At 6 months, and for the positivity of anti-Tg antibodies: values of maternal height and newborn weight were significant (Table 3).
At six moths and for the positivity of anti-TPO antibodies: the number of miscarriages, the newborn weight and the newborn Rh were also significant (Table 4).
Interestingly only two obstetrical parameters were significant during the complete follow up: the newborn weight, which correlated with both antibodies (anti-Tg and anti-TPO) positivity and the maternal height, which exclusively correlated with anti-Tg antibody positivity. We considered that the persistence of correlation throughout the entire study (2 and 6 months) strengthened the association.
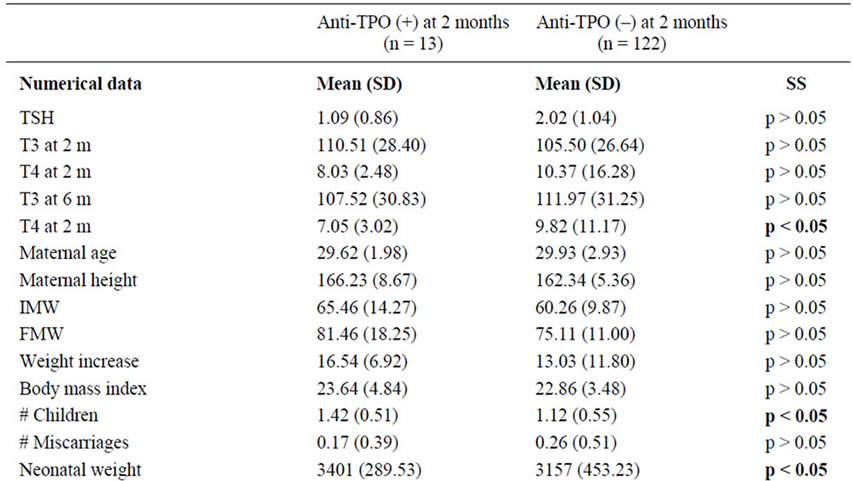

Table 2. Comparison between patients anti-TPO (+) and patients anti-TPO (–) at 2 months of puerperium.

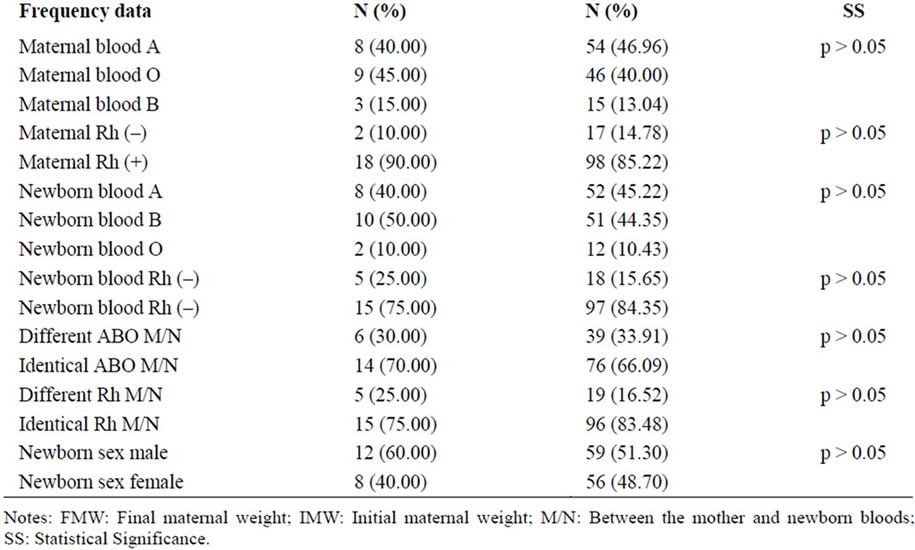
Table 3. Comparison of patients anti-Tg (+) with patients anti-Tg (–) at 6 months of puerperium.
Conversely, other parameters such as TSH, T3, T4, number of children, number of miscarriages, newborn Rh or discrepancy between mother and newborn Rh were only significant for only one antibody and only at one time of the study. We therefore did not consider them to be strongly associated with the postpartum presence of antithyroid antibodies.
4. DISCUSSION
Postpartum thyroiditis etiology is not well understood. It has been related with the appearance of antithyroid antibodies (anti-TG and anti-TPO) [2-11]. But experimental observation shows that the initiation and progression of postpartum thyroiditis in women with positive antibodies is not only a function of their blood level, but is also related with their capability to activate the immune system [23-27]. Th1 and Th2 lymphocytes are also crucial in the pathogenesis of autoimmune thyroiditis: B-lymphocytes need the help of Th2 in order to produce antithyroid antibodies and Th1 lymphocytes are directly involved in thyroid cells’ survival. Also, there is a higher frequency of HLA-DR3, DR4 and DR5 in patients with postpartum thyroiditis [28-32] and leptin, which promotes the development of Th1 [33] presents a higher level in the blood of women with positive anti-TPO antibodies.
It has been suggested that the origin of postpartum thyroiditis could be related with fetal microchimerism (infiltration during pregnancy and puerperium of maternal tissues by fetal haematopoietic or trophoblastic cells). Immune suppression of maternal immunity by the feto placental unit (with production of progesterone and other


Table 4. Comparison of patients anti-TPO (+) with patients anti-TPO (–) at 6 months of puerperium.
immunosuppressive molecules) and the increase of T helper differentiation into Th2, allow a transitory maternal tolerance to such cells. Once fetal cells migrate to maternal tissues, they take advantage of this immune status and survive. However, after delivery, the favourable immunological status ends, and fetal cells start to trigger graft versus host reactions producing antithyroid antibodies.
Therefore, accumulation of fetal cells inside the thyroid gland during pregnancy induces a posterior immunologic disturbance with the development of autoimmune thyroid disease. As fetal cells in maternal tissue have been found even 27 years after delivery, the effect on the immunological system may last for decades [34,35].
Other factors classically associated with the appearance of postpartum thyroiditis have been the iodine intake [36,37], (with contradictory conclusions), smoking [6] and type I diabetes [18,38,39].
In our study, the distinct parameters which turned out to be significant were heterogeneous and with the exception of the newborn weight and the maternal height, in the remaining parameters, statistical significance was not permanent throughout the 6 months follow up, so the correlations were not conclusive.
The higher newborn weight in patients with positive anti-TPO and anti-Tg might be related to a significantly higher increase in maternal weight during pregnancy. However in our study, maternal weight increase showed correlation only with anti-Tg at 2 months, while newborn weight maintained correlation for both antibodies at 2 and six months, showing that its positivity could not be explained only according to the well known relationship between neonatal weight and maternal weight increase. Therefore, the persistence of significance for neonatal weight indicated a possible relationship between neonatal weight and the appearance of antithyroid antibodies.
The cause of postpartum thyroiditis has been related with the existence of microchimerism (appearance of fetal cells in maternal tissues) [34], as a higher newborn weight supposes a higher number of fetal cells, the bigger fetal size supposes a higher possibility of fetal cells transfer to the mother´s tissues and especially to the thyroid gland. In the same manner, the persistence of significance for maternal height during the 6 month follow up in relation with the positivity of the anti-Tg antibody, makes us consider a certain influence of this parameter in the initiation of this disorder. In fact, the bigger the mother is, the bigger the thyroid gland is supposed to be and a higher possibility of fetal cells transfer to the mother’s thyroid gland occurs. Both mechanisms: increased number of fetal cells and increased volume of maternal thyroid gland, raise the probability of fetal cells appearance in thyroid tissue and support the theory which explains development of postpartum thyoriditis as a consequence of fetal microchimerism. The relation between presence of fetal cells in maternal thyroid tissue and development of antithyroid antibodies has become an attractive model for the explanation of postpartum thyroiditis. Interestingly, our study fits within this model as the clinical parameters involved with a higher possibility of fetal microchimerism obtain the strongest correlation with the appearance of postpartum antithyroid antibodies. Although the number of patients with positive antithyroid antibodies was small, the association of their positívity throughout the whole period of study increased the strength of this association.
We recognize these results are preliminary. In spite of that, they might be used as a basis in the search of a postpartum thyroiditis screening. However, in order to develop it, a more detailed analysis will be required, including assessment of a higher number of cases and amplification of the patients’ follow up period.
5. ACKNOWLEDGEMENTS
We thank Miss. Victoria Guerrero (student of Florida State University, USA) for her technical support in reviewing the English translation of this manuscript.
REFERENCES
- Roti, E. and Emerson, C.H. (1992) Clinical review 29: Postpartum thyroiditis. Journal of Clinical Endocrinology & Metabolism, 74, 3-5. doi:10.1210/jc.74.1.3
- Jansson, R., Bernander, S., Karlsson, A., Levin, K. and Nilsson, G. (1984) Autoimmune thyroid dysfunction in the postpartum period. Journal of Clinical Endocrinology & Metabolism, 58, 681-687. doi:10.1210/jcem-58-4-681
- Fung, H.Y., Kologlu, M., Collison, K., John, R., Richerds, C.J., Hall, R. and McGregor, A.M. (1988) Postpartum thyroid dysfunction in mid glamorgan. British Medical Journal, 296, 241-244. doi:10.1136/bmj.296.6617.241
- Amino, N., Mori, H., Iwatani, Y., Tanizawa, O., Kawashima, O., Tsuge, I, Ibaragi, K. Kumahara, Y. and Miyai, K. (1982) High prevalence of transient post-partum thyrotoxicosis and hypothyroidism. The New England Journal of Medicine, 306, 849-852. doi:10.1056/NEJM198204083061405
- Freeman, R., Rosen, H. and Thysen, B. (1986) Incidence of thyroid dysfunction in an unselected postpartum population. Archives of Internal Medicine, 146, 1361-1364. doi:10.1001/archinte.1986.00360190137019
- Hayslip, C.C., Fein, H.G., O’Donnell, V.M., Friedman, D.S., Klein, T.A. and Smallridge, R.C. (1988) The value of serum antimicrosomal antibody testing in screening for symptomatic postpartum thyroid dysfunction. American Journal of Obstetrics & Gynecology, 159, 203-209.
- Kuijpens, J.L., De Hann-Meulman, M., Vader, H.L., Pop, V.J., Wiersinga, W.M. and Drexhage, H.A. (1998) Cellmediated immunity and postpartum thyroid dysfunction: A possibility for the prediction of disease? Journal of Clinical Endocrinology & Metabolism, 83, 1959-1966. doi:10.1210/jc.83.6.1959
- Lervang, H.H., Pryds, O. and Ostergaard Kristensen, H.P. (1987) Thyroid dysfunction after delivery: Incidence and clinical course. Acta Medica Scandinavica, 222, 369-374. doi:10.1111/j.0954-6820.1987.tb10685.x
- Pop, V.J., De Rooy, H.A., Vader, H.L., Van Der Heide, D., Van Son, M.M. and Komproe, I.H. (1993) Microsomal antibodies during gestation in relation to postpartum thyroid dysfunction and depression. Acta Endocrinologica (Copenhagen), 129, 26-30.
- Stagnaro-Green, A., Roman, S.H., Cobin, R.H., ElHarazy, E., Wallenstein, S. and Davies, T.F. (1992) A prospective study of lymphocyte-initiated immunosuppression in normal pregnancy: Evidence of a T-cell etiology for postpartum thyroid dysfunction. Journal of Clinical Endocrinology & Metabolism, 74, 645-653. doi:10.1210/jc.74.3.645
- Sakaihara, M., Yamada, H., Kato, E.H., Ebina, Y., Shimada, S., Kobashi, G., Fukushi, M. and Fujimoto, S. (2000) Postpartum thyroid dysfunction in women with normal thyroid function during pregnancy. Clinical Endocrinology, 53, 487-492. doi:10.1046/j.1365-2265.2000.01107.x
- Harris, B., Othman, S., Davies, J.A., Weppner, G.J., Richards, C.J., Newcombe, R.G., Lazarus, J.H., Parkes, A.B., Hall, R. and Phillips, D.I. (1992) Association between postpartum thyroid dysfunction and thyroid antibodies and depression. British Medical Journal, 305, 152-156. doi:10.1136/bmj.305.6846.152
- Kent, G.N., Stuckey, B.G., Allen, J.R., Lambert, T. and Gee, V. (1999) Postpartum thyroid dysfunction: Clinical assessment and relationship to psychiatric affective morbidity. Clinical Endocrinology, 51, 429-438. doi:10.1046/j.1365-2265.1999.00807.x
- Lucas, A., Pizarro, E., Granada, M.L., Salinas, I., Foz, M. and Sanmarti, A. (2000) Postpartum thyroiditis: Epidemiology and clinical evolution in a nonselected population. Thyroid, 10, 71-77. doi:10.1089/thy.2000.10.71
- Nikolai, T.F., Turney, S.L. and Roberts, R.C. (1987) Postpartum lymphocytic thyroiditis. Prevalence, clinical course, and long-term follow-up. Archives of Internal Medicine, 147, 221-224. doi:10.1001/archinte.1987.00370020041032
- Rajatanavin, R., Chailurkit, L.O., Tirarungsikul, K., Chalayondeja, W., Jittivanich, U. and Puapradit, W. (1990) Postpartum thyroid dysfunction in Bangkok: A geographical variation in the prevalence. Acta Endocrinol (Copenh), 122, 283-287.
- Rasmussen, N.G., Hornnes, P.J., Hoier-Madsen, M., Feldt-Rasmussen, U. and Hegedus, L. (1990) Thyroid size and function in healthy pregnant women with thyroid autoantibodies. Relation to development of postpartum thyroiditis. Acta Endocrinologica (Copenhagen), 123, 395-401.
- Roti, E., Bianconi, L., Gardini, E., Minelli, R., De Franco, M.L., Bacchi Modena, A., Bresciani, D., Villa, P., Neri, T.M. and Savi, M. (1991) Postpartum thyroid dysfunction in an Italian population residing in an area of mild iodine deficiency. Journal of Endocrinological Investigation, 14, 669-674.
- Vargas, M.T., Briones-Urbina, R., Gladman, D., Papsin, F.R. and Walfish, P.G. (1988) Antithyroid microsomal autoantibodies and HLA-DR5 are associated with postpartum thyroid dysfunction: Evidence supporting an autoimmune pathogenesis. Journal of Clinical Endocrinology & Metabolism, 67, 327-333. doi:10.1210/jcem-67-2-327
- Walfish, P.G., Meyerson, J., Provias, J.P., Vargas, M.T. and Papsin, F.R. (1992) Prevalence and characteristics of post-partum thyroid dysfunction: Results of a survey from Toronto, Canada. Journal of Endocrinological Investigation, 15, 265-272.
- Barca, M.F., Knobel, M., Tomimori, E., Cardia, M.S. and Medeiros-Neto, G. (2000) Prevalence and characteristics of postpartum thyroid dysfunction in Sao Paulo, Brazil. Clinical Endocrinology, 53, 21-31. doi:10.1046/j.1365-2265.2000.01034.x
- Kessler, S. (1995) De la analítica al diagnóstico. Iatros, Barcelona, 144-147.
- Parkes, A.B., Othman, S., Hall, R., John, R., Richards, C.J. and Lazarus, J.H. (1994) The role of complement in the pathogenesis of postpartum thyroiditis. Journal of Clinical Endocrinology & Metabolism, 79, 395-400. doi:10.1210/jc.79.2.395
- Parkes, A.B., Othman, S., Hall, R., John, R. and Lazarus, J.H. (1995) Role of complement in the pathogenesis of postpartum thyroiditis: Relationship between complement activation and disease presentation and progression. European Journal of Endocrinology, 133, 210-215. doi:10.1530/eje.0.1330210
- Jansson, R., Thompson, P.M., Clark, F. and McLachlan, S.M. (1986) Association between thyroid microsomal antibodies of subclass IgG-1 and hypothyroidism in autoimmune postpartum thyroiditis. Clinical & Experimental Immunology, 63, 80-86.
- Pinchera, A., Ingbar, S.H., McKenzie, J.M. and Fenzi G.F. (1988) Thyroid autoimmunity. Plenum Press, New York.
- Weetman, A.P., Fung, H.Y., Richards, C.J. and McGregor, A.M. (1990) IgG subclass distribution and relative functional affinity of thyroid microsomal antibodies in postpartum thyroiditis. European Journal of Clinical Investigation, 20, 133-136. doi:10.1111/j.1365-2362.1990.tb02259.x
- Emerson, C.H. (1991) Thyroid disease during and after pregnancy. In: Braverman, L.E. and Utiger, R.D., Eds., The Thyroid, JB Lippencott Co., Philadephia.
- Farid, N.R., Hawe, B.S. and Walfish, P.G. (1983) Increased frequency of HLA-DR3 and 5 in the syndromes of painless thyroiditis with transient thyrotoxicosis: Evidence for an autoimmune aetiology. Clinical Endocrinology, 19, 699-704. doi:10.1111/j.1365-2265.1983.tb00047.x
- Tachi, J., Amino, N., Tamaki, H., Aozasa, M., Iwatani,Y. and Miyai, K. (1988) Long term follow-up and HLA association in patients with postpartum hypothyroidism. Journal of Clinical Endocrinology & Metabolism, 66, 480-484. doi:10.1210/jcem-66-3-480
- Lervang, H.H., Pyrds, O., Kristensen, H.P., Jakobsen, B.K. and Svejgaard, A. (1984) Postpartum autoimmune thyroid disorder associated with HLA-DR4? Tissue Antigens, 23, 250-252. doi:10.1111/j.1399-0039.1984.tb00040.x
- Jansson, R., Safwenberg, J. and Dahlberg, P.A. (1985) Influence of the HLA-DR4 antigen and iodine status on the development of autoimmune postpartum thyroiditis. Journal of Clinical Endocrinology & Metabolism, 60, 168-173.
- Mazziotti, G., Parkes, A.B., Lage, M., Premawardhana, L.D., Casanueva, F.F. and Lazarus, J.H. (2004) High leptin levels in women developing postpartum thyroiditis. Clinical Endocrinology, 60, 208-213. doi:10.1046/j.1365-2265.2003.01966.x
- Imaizumi M., Pritsker A., Unger, P. and Davies, T.F. (2002) Intrathyroidal fetal microchimerism in pregnancy and postpartum. Journal of Clinical Endocrinology & Metabolism, 143, 247-253.
- Ando, T. and Davies, T.F. (2003) Clinical review 160: Postpartum autoimmune thyroid disease: The potential role of fetal microchimerism. Journal of Clinical Endocrinology & Metabolism, 88, 2965-2971. doi:10.1210/jc.2002-021903
- Kampe, O., Jansson, R. and Karlsson, F.A. (1990) Effects of L-thyroxine and iodide on the development of autoimmune postpartum thyroiditis. Journal of Clinical Endocrinology & Metabolism, 70, 1014-1018. doi:10.1210/jcem-70-4-1014
- Nøhr, S.B., Jorgensen, A., Pedersen, K.M. and Laurberg, P. (2000) Postpartum thyroid dysfunction in pregnant thyroid peroxidase antibody-positive women living in an area with mild to moderate iodine deficiency: Is iodine supplementation safe? Journal of Clinical Endocrinology & Metabolism, 85, 3191-3198. doi:10.1210/jc.85.9.3191
- Lazarus, J.H. (1998) Prediction of postpartum thyroiditis. European Journal of Endocrinology, 139, 12-13. doi:10.1530/eje.0.1390012
- Lazarus, J.H. (1999) Clinical manifestations of postpartum thyroid disease. Thyroid. European Journal of Endocrinology, 9, 685-689.

