Paper Menu >>
Journal Menu >>
 J. Biomedical Science and Engineering, 2011, 4, 214-218 JBiSE doi:10.4236/jbise.2011.43030 Published Online March 2011 (http://www.SciRP.org/journal/jbise/). Published Online March 2011 in SciRes. http://www.scirp.org/jour nal/JBiSE The diagnostic significance and the assessment of the value of vascular endothelial growth factor as a marker for success of chemical pleurodesis in malignant pleural effusion Dalokay Kilic1, Alper Findikcioglu1, Goknur Alver2, Tolga Tatar1, Hakan Akbulut3, Ahmet Hatipoglu4 1Department of Thoracic Surgery, Baskent University, School of Medicine, Baskent University Hospital, Ankara, T urkey; 2Department of Thoracic Surgery, Ataturk Hospital, Ankara, Turkey; 3Department of Medical Oncology, Ankara University, School of Medicine, Ankara, Turkey; 4Department of Thoracic Surgery, Ankara University, School of Medicine, Ankara, Turkey. Email: dalokay7@yahoo.com Received 20 November 2009; revised 12 December 2009; accepted 16 December 2009. ABSTRACT Differential diagnosis of pleural effusion is an im- portant issue, since the treatment modalities and prognosis strictly depend on early and correct diag- nosis of the underlying etiology. We assessed the effi- cacy of vascular endothelial growth factor (VEGF) in the differential diagnosis of patients with malignant and non-malignant pleural diseases. And also is as- sessed of the VEGF as a marker for success of chemi- cal pleurodesis in malignant pleural effusion. Pleural effusions of 40 pat ients with a mean age of 55 (range, 26 to 78 years) were examined. A total of 20 patients had malig nant pleural eff usion; malig nant meso- t he- lioma (n = 7), lung cancer (n = 5) and metastatic ma- lignancies (n = 8). Twenty patients had benign pleural effusion; fibrinous pleuritis (n = 6), tubercu- losis (n = 3) empyema (n = 5), congestive heart failure (n = 3), and acute pancreatitis (n = 3). Definitive di- agnosis was obtained in all cases with blind or open pleural biopsy, and cytological examination. VEGF levels were determined by enzyme-linked immu- nosorbent assay. The VEGF level of pleural effusion was com- parably higher in the malignant group. The mean level of VEGF in patients with malignant pleu- ral effusions (21.7 ± 1.8 ng/ml) was significantly (P < 0.001) higher than that of (13.2 ± 1.5 ng/ml) non-ma- lignant effusions. No significant difference was found regarding the VEGF levels and histological types in malignant pleural effusions. Negative correlation was observed between success rate of pleurodesis and VEGF level of pleural effusion (p = 0.015). The measurement of VEGF levels in pleural effusion may be useful to differentiate malignant from nonmalig- nant pleural effusions. VEGF level may also be an important prognostic marker for ef- fective treatment of the patients who had malignant pleural effusions with pleurodesis. It is important issue in here whether VEGF could be useful in prog- nostication of outcome of chemical pleurodesis or not. Keywords: Malig nant Pleural Effusion; Pleural Effusion; Chemical Pleurodesis; Vascularendothelial Growth Factor 1. INTRODUCTION Pleural effusion is an important problem in malign ant or non-malignant pleural disease, causing severe symptoms such as dyspnea and chest pain. Management of the pleural effusion (PE) depends on the underlying etiology. Inflammatory PE can be treated easily with success, contrary to malignant PE, in which the main goal is to decrease symptoms and increase the quality of life as much as possible. This “mandatory” differential diagno- sis is still difficult, time-consuming and expensive. Pleural fluid accumulation in malignancy is generally believed to be secondary to lymphatic obstruction by malignant cells [1]. However recent studies pointed to vascular endothelial growth factor (VEGF) as a key agent in this entity [2,3]. VEGF, produced by malignant pleural tissue, is thought to both enhance tumor angio- genesis leading to local growth, and increase vascular permeability leading to PE [2,3]. We conducted a study to investigate th e role of VEGF in differentiating between malignant and non-malignant pleural effusions in a series of 40 patients. 2. MATERIAL AND METHODS 2.1. Materials We measured the VEGF levels of pleural effusions in 40 patients consisting of 24 (60%) male and 16 (40%) fe- male patients with a mean age of 54.5 years (range, 26 to  D. Kilic et al. / J. Biomedical Science and Engineering 4 (2011) 214-218 Copyright © 2011 SciRes. JBiSE 215 78 years) by enzyme-linked immunosorbent assay (ELISA). Twenty patients had malignant pleural effusion including malignant mesothelioma (n = 7), lung cancer (n = 5), metastasis from genitourinary system malignan- cies (renal cell Ca, endometrium Ca, ovarian Ca) (n = 3), metastasis from breast carcinoma (n = 3), adenocarci- noma metastasis from gastrointestinal system (n = 1), Non Hodgkin-lymphoma (n = 1). Twenty patients had benign pleural effusion associated with fibrinous pleu- ritis (n = 6), tuberculosis (n = 3) empyema (n = 5), con- gestive heart failure (n = 3), and acute pancreatitis (n = 3). Definitive diagnosis was obtained in all patients with either open or blind pleural biopsies and cytological examination (Table 1). 2.2. Analysis of Material Properties and Invitro Study VEGF concentrations were measured using an enzyme- linked immunosorbent assay (ACCUCYTE®, assay sys- tem, Human VEGF ELISA kit, Cytimmune Sciences, Inc. Maryland USA). Tecnique of sampling and storage of PE: Pleural effusion was collected in a sterile centrifuge tube and centifugated at 3000 rpm for 10 min at 4˚C. The cell-free supernatant was then separated and stored immediately at –70˚C until assayed for VEGF. VEGF level in PE measured in duplicate for each sample with an ELISA kit that recognizes the soluble isoform VEGF 165. This assay is sensitiv e to 0.195 ng/ml. The kit used for the detection of total (bound and unbound) VEGF is designated to capture a specific VEGF complex consist- ing of VEGF antibody, biotinyl-ated VEGF and sam- ple/standard. The optical density was measured at 492 Table 1. Definitive diagnosis and VEGF levels in patients with pleural effusions. Malignant effusion n/Mean VEG F level (ng/ml) Nonmalignant effusion n/Mean VEG F level (ng/ml) Malignant mesothelioma 7 (20.7 ± 3.8) Fibrinous Pleurit 6 (12.8 ± 2.1) Lung Cancer (ADC*) 5 (24.2 ± 2.8) Empyema 5 (20.8 ± 1.8) Metastatic Malignancies 8 (20. 9 ± 3.6) Congestive Heart Failure 3 (7.3 ± 1.2) Breast Cancer 3 Acute Pancreatitis 3 (12.1 ± 4.4) Metastasis of GUS** Cancer 3 Tuberculosis 3 (8.8 ± 2.7) Metastasis of GIS*** ADC 1 - - Non-Hodgkin’s Lymphoma 1 - - Total 20 (21.7 ± 1.8) - 20 (13.2 ± 1.5) ADC*. Adenocarcinoma; GUS**. Genitourinary system; GIS***. Gastro- intestinal system nm. The glucose and lactate dehydrogenase (LDH) meas- urements were obtained from fluid collection in a sterile tube using an automated analyzer. 2.3. Statistical Analysis Statistical comparisons of baseline data between groups were performed by the Mann-Whitney U test as appro- priate. Data were considered statistically significant if P values were less than 0.05. Data were expressed as mean ± the standard deviation (SD). Correlations were ana- lyzed with the Spearman rank order correlation. All sta- tistical analyses were performed with the Statistical Package for the Social Sciences (version 11.0; SPSS, Inc., Chi c ago, Illin ois, USA). 3. RESULTS 3.1. Definitive Diagnosis and VEGF Levels in Patients with Pleural Effusions Twenty patients had malignant pleural effusion associ- ated with malignant mesothelioma (20.7 ± 3.8 ng/ml), lung cancer (24.2 ± 2.8 ng/ml), metastasis from genitou- rinary system carcinomas (renal cell Ca, endometrium Ca, ovarian Ca) (21.3 ± 6.9 ng/ml) metastasis from breast carcinoma (19.7 ± 4.3 ng/ml), adenocarcinoma metasta- sis from gastrointestinal system (23.8 ng/ml), and Non- Hodgkin’s Lymphoma (21.1 ng/ml). Twenty patients had benign pleural effusion associated with fibrinous pleu- ritis (12.8 ± 2.1 ng/ml), tuberculosis (8.8 ± 2.9 ng/ml) empyema (20.8 ± 1.8 ng/ml), congestive heart failure (7.3 ± 1.2 ng/ml), and acute pancreatitis (12.1 ± 4.4 ng/ml) (Table 1). 3.2. Laboratory Results The mean level of VEGF in patients with malignant pleural effusions (21.7 ± 1.8 ng/ml) was significantly higher than that of non-malignant (13.2 ± 1.5 ng/ml) pleural effusions (p < 0.001) (Figure 1). No significant differences were observed in concentration of pleural VEGF in different histological types of malignant pleu- ral effusion (malignant mesothelioma, lung cancer (p = 0.482) and malignant mesothelioma, other metastatic car- cinomas (excluding lung cancer) (p = 0.354). Mean LDH level of PE was 614.32 288.62 IU/L and mean glucose level of PE was 77.0 30.6 mg/dL. The closest correla- tion was between the pleural effusion VEGF level and the LDH level (r = 0.894, p < 0.001) ( Figure 2). No sig- nificant correlation existed between the pleural effusion VEGF level and the glucose level (r = 0.079, p = 0.628). 3.3 Comparison of Success of Chemical Pleurodesis and VEGF Level Seventeen patients who had malignant pleural effusion underwent chemical pleurodesis. Five of the patients 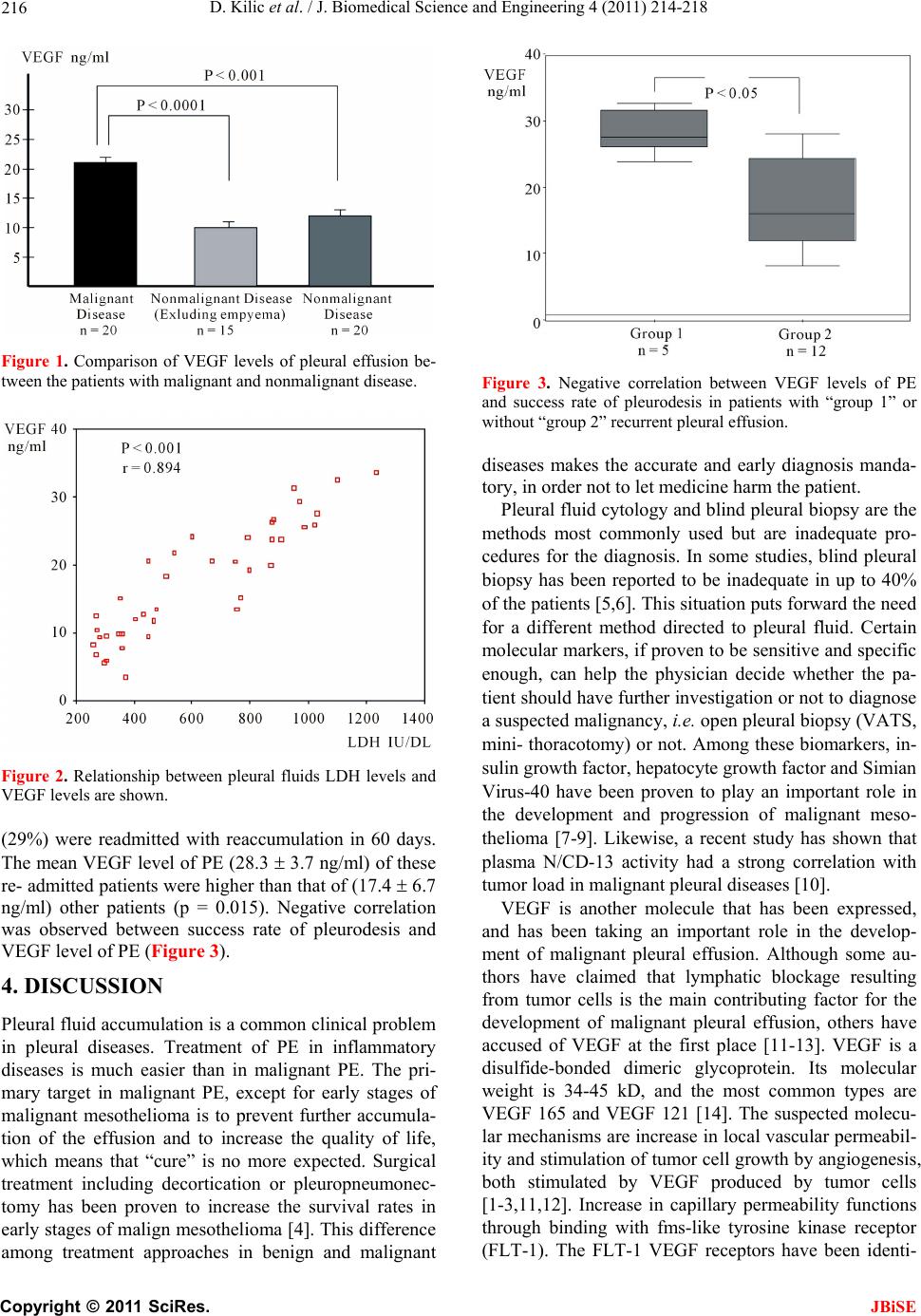 D. Kilic et al. / J. Biomedical Science and Engineering 4 (2011) 214-218 Copyright © 2011 SciRes. JBiSE 216 Figure 1. Comparison of VEGF levels of pleural effusion be- tween the patients with malignant and nonmalignant disease. Figure 2. Relationship between pleural fluids LDH levels and VEGF levels are shown. (29%) were readmitted with reaccumulation in 60 days. The mean VEGF level of PE (28.3 3.7 ng/ml) of these re- admitted patients were higher than th at of (17.4 6.7 ng/ml) other patients (p = 0.015). Negative correlation was observed between success rate of pleurodesis and VEGF level of PE (Figure 3). 4. DISCUSSION Pleural fluid accumulation is a common clinical problem in pleural diseases. Treatment of PE in inflammatory diseases is much easier than in malignant PE. The pri- mary target in malignant PE, except for early stages of malignant mesothelioma is to prevent further accumula- tion of the effusion and to increase the quality of life, which means that “cure” is no more expected. Surgical treatment including decortication or pleuropneumonec- tomy has been proven to increase the survival rates in early stages of malign mesothelioma [4]. This difference among treatment approaches in benign and malignant Figure 3. Negative correlation between VEGF levels of PE and success rate of pleurodesis in patients with “group 1” or without “group 2” recurrent pleural effusion. diseases makes the accurate and early diagnosis manda- tory, in order not to let medicine harm the patient. Pleural fluid cytology and blind pleural biopsy are the methods most commonly used but are inadequate pro- cedures for the diagnosis. In some studies, blind pleural biopsy has been reported to be inadequate in up to 40% of the patien ts [5,6]. Th is situatio n puts forward the n eed for a different method directed to pleural fluid. Certain molecular markers, if proven to be sensitiv e and specific enough, can help the physician decide whether the pa- tient should have further investigation or not to d iagnose a suspected malignancy, i.e. open pleural biopsy (VATS, mini- thoracotomy) or not. Among these biomarkers, in- sulin growth fa ct or, hepatocyte gro wt h facto r and Simian Virus-40 have been proven to play an important role in the development and progression of malignant meso- thelioma [7-9]. Likewise, a recent study has shown that plasma N/CD-13 activity had a strong correlation with tumor load in malignant pleural diseases [10]. VEGF is another molecule that has been expressed, and has been taking an important role in the develop- ment of malignant pleural effusion. Although some au- thors have claimed that lymphatic blockage resulting from tumor cells is the main contributing factor for the development of malignant pleural effusion, others have accused of VEGF at the first place [11-13]. VEGF is a disulfide-bonded dimeric glycoprotein. Its molecular weight is 34-45 kD, and the most common types are VEGF 165 and VEGF 121 [14]. The suspected molecu- lar mechanisms are increase in local vascular permeabil- ity and stimulation of tumor cell growth by angiogenesis, both stimulated by VEGF produced by tumor cells [1-3,11,12]. Increase in capillary permeability functions through binding with fms-like tyrosine kinase receptor (FLT-1). The FLT-1 VEGF receptors have been identi-  D. Kilic et al. / J. Biomedical Science and Engineering 4 (2011) 214-218 Copyright © 2011 SciRes. JBiSE 217 fied in pleural mesothelial cells and vascular endothelial cells also high densities in infiltrating malignant tissue [13]. It has been shown to be important regulatory sys- tems for angiogenesis and vasculogenesis [12]. Many studies have shown that several types of tumor cells express VEGF. VEGF is an important cytokine in lung cancer [15,16 ] Matsuyama et al. reported a positive correlation between serum VEGF and stage progression of the disease [15]. Measurement of serum VEGF levels was suggested to be useful to evaluate lung cancer pro- gression. Similar data are obtained from studies directed to gastric, colorectal and renal cell carcinomas [17-19]. It also seems to be an important determinant in malign- nant pleural disease. Tickett et al. have reported that me- dian VEGF levels of 2500 pg/ml in malignant PE were significantly higher than of 305pg/ml in the non-malig- nant group [13]. Our study is in accordance with these previous data, showing an increased level of VEGF in malignant effusion. Lim et al. compared VEGF levels of tuberculous PE (median, 994 pg/ dl) and malignant PE (2418 pg/ dl), reaching to similar results obtained in our study [20]. In our se ries, VEGF of PE levels in empyem a patients were higher than other nonmalignant groups (p = 0.02). There were no significant differences between VEGF levels in empyema and in lung cancer (p = 0.349). Similar results have been reported by Thickett [13]. The known biological functions of VEGF may promote the accumulation of pleural fluid and increase the loculation and organization of empyema [21]. In our study, when VEGF levels found in empyema cases were excluded, the levels were more significantly higher in malignant than in non-malignan t PE (P < 0.0001) similar to Thick- ett’s study [13]. If patients having high levels of VEGF in suspicious PE that cannot be diagnosed with blind biopsy of the pleura or cytological techniques, patients should undergo an early open biopsy. Thickett et al. reported false nega- tive rate of 50 % in the malignant group after blind bi- opsy with a median level of VEGF higher than the level in the benign group. However, in the abovementioned study, additional invasive procedures were needed for de finitive diagnosis [13]. In our study, the results obtained via pleural fluid cytology in addition to blind biopsy were false negative in 25% (n = 5) of the malignant group mean with a mean VEGF level of PE = 21.2 ± 1.4 ng/ml and definitive diagnosis had to be made by VATS and mini-thoracotomy. VEGF and LDH in pleural effusion correlated because they are both crude markers of the inflammatory re- sponse [22]. A new experimental study for the treatment of PE in- cludes VEGF receptor (receptor tyrosine kinase) block- age model for human tumors [23,24]. VEGF receptor blockage model may also provide a new therapeutic ap- proach for pleural malignancies. Further studies are nec- essary to outline the feasibility of VEGF receptor block- age model as a therapeutic modality. As a conclusion, malignant pleural effusions show significantly higher VEGF levels compared with non- malignant pleural effusions. Thus, assessment of VEGF levels may be used to differentiate malignant from non- malignant pleural effusions as an adjunct to conventional differential diagnostic techn iques. Low lev el of VEGF in malignant PE patients may be a good prognostic marker for effective treatment of malignant PE with pleurodesis. REFERENCES [1] Sahn, S.A. (1997) Pleural diseases related to metastatic malignancies. European Respiratory, 10, 1907-1913. doi:10.1183/09031936.97.10081907 [2] Fontanini, G., Vignati, S., Boldrini L., Chinè, S., Silvestri, V., Lucchi, M., Mussi, A., Angeletti, C.A. and Bevilac- qua, G. (1997) Vascular endothelial growth factor is as- sociated with neovascularization and influences progres- sion of non-small cell lung carcinoma. Cliical Cancer Research, 3, 861-865. [3] Yanagawa, H., Takeuchi, E., Suzuki, Y., Ohmoto, Y., Bando, H. and Sone, S. (1999) Vascular endothelial growth factor in with malignant pleural effusion associ- ated with lung cancer. Cancer Immunology Immuno- therapy, 48, 396-400. doi:10.1007/s002620050592 [4] Rusch, V.W. and Venkatraman, E.S. (1999) Important prognostic factors in patients with malignant pleur al meso- thelioma, managed surgically. Annalls of Thoraic Surgery, 68, 1799-1804. doi:10.1016/S0003-4975(99)01038-3 [5] Poe, R.H., Isreal, R.H. and Utell, M.J. (1984) Sensitivity, spesificity, and predictive values of closed pleural biopsy. Arcives of Internal Medicine, 144, 325-328. doi:10.1001/archinte.144.2.325 [6] Sahn, S.A. (1988) The pleura. The American Review of Respiratory Disease, 138, 184-234. [7] Lee, T.C.Y., Zhang, C., Aston R., Hintz, R., Jagirdar, J., Perle, M.A., Burt, M. and Rom, W.N. (1993) Normal hu- man mesothelial cells and mesothelioma cell lines ex- press insulin like growth factor I and associated mole- cules. Cancer Research, 53, 2858-2864. [8] Harvey, P., Warn, S.A., Dobbin, S., Arakaki, N., Daiku- hara, Y., Jaurand, M.C. and Warn, R.M. (1998) Expres- sion of HGF/SF in mesothelioma cell lines and its effects on cell motility, proliferation and morphology. British Journal of Can cer, 77, 1052-1059. doi:10.1038/bjc.1998.176 [9] Cacciotti, P., Strizzi, L., Vianale, G., Iaccheri, L., Libener, R., Porta, C., Tognon, M., Gaudino, G. and Mutti, L. (2002) The presence of simian-virus 40 sequences in mesothelioma and mesothelial cells is associated with high levels of vascular endothelial growth factor. Ameri- can Journal of Respirartory Cell and Molecular Biology, 26, 189-193. [10] Hensbergen, Y.V., Broxterman, H.J., Hanamaaijer, R., Jorna, A.S., van Lent, N.A., Verheul, H. M., Pi nedo, H.M. and Hoekman, K. (2002) Soluble aminopeptida se N/CD13 in malignant and nonmalignant effusion and intratumoral  D. Kilic et al. / J. Biomedical Science and Engineering 4 (2011) 214-218 Copyright © 2011 SciRes. JBiSE 218 fluid. Clinical Cancer Research, 8, 3747-3754. [11] Yano, S., Shinohara, H., Herbst, R.S., Kuniyasu, H., Bu- cana, C.D., Ellis, L.M. and Fidler, I.J (2000) Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular en- dothelial growth factor/vascular permeability factor by human lung cancer cells. American Journal of Pathol- ogy, 157, 1893-1903. doi:10.1016/S0002-9440(10)64828-6 [12] Strizzi, L., Catalano, A., Vianale, G., Orecchia, S., Casal- ini, A., Tassi, G., Puntoni, R., Mutti, L. and Procopio, A. (2001) Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. Jour- nal of Pathology, 193, 468-475. doi:10.1002/path.824 [13] Thickett, D.R., Armstrong, L. and Millar, A.B. (1999) Vascular endothelial growth factor (VEGF) in inflamma- tory and malignant pleural effusions. Thorax, 54, 707-710. doi:10.1136/thx.54.8.707 [14] Ferrara, N. (1999) Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med, 77, 527-543. doi:10.1007/s001099900019 [15] Matsuyama, W., Hashiguchi, T., Mizoguchi, A., Iwami, F., Kawabata, M., Arimura, K. and Osame, M. (2000) Serum levels of vascular endothelial growth factor de- pendent on the stage progression of lung cancer. Chest, 118, 948-951. doi:10.1378/chest.118.4.948 [16] Mattern, J., Koomagi, R. and Volm, M. (1997) Coexpres- sion of VEGF and bFGF in human epidermoid lung car- cinoma is associated with increased vessel density. Anti-cancer Research, 17, 2249-2252. [17] Eroglu, A., Demirci, S., Ayyildiz, A., Kocaoglu, H., Akbulut, H., Akgul, H. and Elhan, H.A. (1999) Serum concentration of vascular endothelial growth factor and nitrite as an estimate of in vivo nitric oxide in patients with gastric cancer. British ournal of. Cancer, 80, 1630-1634. doi:10.1038/sj/bjc/6690573 [18] Akbulut, H., Altuntas, F., Akbulut, K.C., Ozturk, G., Cindoruk, M., Unal, E. and Icli, F. (2002) Prognostic role of serum vascular endothelial growth factor, basic fibro- blast growth and nitrit oxide in patients with colorectel carcinoma. Cytok ine, 20, 184-190. doi:10.1006/cyto.2002.1993 [19] Paradise, V., Lagha, N.B. and Zeimoura, L. (2000) Ex- pression of vascular endothelial growth factor in renal cell carcinomas. Vir ch ows A rchiv, 436, 351-356. doi:10.1007/s004280050458 [20] Lim, S.C., Jung, S.I., Kim, Y.C. and Park, K.O. (2000) Vascular endothelial growth factor in malignant and tu- berculous pleural effusions. Journal of Korean Medicine Science, 15, 279-283. [21] Nehls, V. and Herrmann, R. (1998) The configuration of fibrin clots determines capillary morphogenesis and endo- thelial cell migration. Microvascular Research, 2, 9-20. [22] Taichman, N.S., Young, S., Cruchley, A.T., Taylor, P. and Paleolog, E. (1997) Human neutrophils secrete vascular endothelial growth factor. Journal of Leukocyte Biology, 62, 397-400. [23] Masood, R., Tong, Zheng, J.C., Smith, D.L., Hinton, D.R. and Gill, P.S. (2001) Vascular endothelial growth factor (VEGF) is an autocrine growth factor for VEGF recep- tor-positive human tumors. Blood, 98, 1904-1913. doi:10.1182/blood.V98.6.1904 [24] Verheul, H.M.W., Hoekman, K., Jorna, A.S., Smit, E.F. and Pinedo, H.M. (2000) Targeting vascular endothelial growth factor blockade: Ascites and pleural effusion formation. The Oncologist, 5, 45-50. doi:10.1634/theoncologist.5-suppl_1-45 |

