Journal of Biomedical Science and Engineering
Vol. 5 No. 11 (2012) , Article ID: 24675 , 8 pages DOI:10.4236/jbise.2012.511079
Poly(N-isopropylacrylamide-co-N-tert-butylacrylamide)- grafted hyaluronan as an injectable and self-assembling scaffold for cartilage tissue engineering
![]()
Division of Life Science and Engineering, School of Science and Engineering, Tokyo Denki University, Saitama, Japan
Email: k-muramatsu@mail.dendai.ac.jp
Received 10 August 2012; revised 18 September 2012; accepted 30 September 2012
Keywords: Thermally Responsive Material; Cartilage Tissue Engineering; Biocompatibility; Mesenchymal Cells
ABSTRACT
Novel poly(N-isopropylacrylamide-co-N-tert-butylacrylamide)-grafted hyaluronan [P(NIPAAm-co-NtBAAm)- g-HA] has been developed as a modified derivative to improve phase-transition characteristics of PNIPAAmg-HA, which has a lower critical solution temperature (LCST) of approximately 32˚C. This promising selfassembling biomaterial has potential as an injectable scaffold for in situ cartilage tissue engineering. LCST of the P(NIPAAm-co-NtBAAm)-g-HA decreased to approximately 3.6˚C compared to that of the original PNIPAAm-g-HA. This modification enabled self-assembly at body temperatures lower than the temperature of the parental PNIPAAm-g-HA molecule. Cytotoxicity and acute systemic toxicity assays revealed that P(NIPAAm-co-NtBAAm)-g-HA was not hazardous. The DNA content of chondrogenic differentiated mesenchymal stem/stromal cells (MSCs) embedded in the gels was higher than that of biomaterial-free aggregates during the culture periods. Cartilage-related genes were also expressed in chondrogenic differentiated MSCs embedded in the P (NIPAAm-co-NtBAAm)-g-HA hydrogel. Specifically, an increased expression of SRY-related HMG boxcontaining gene 9 (Sox9) observed in the hydrogel group compared to controls. These data suggest that P(NIPAAm-co-NtBAAm)-g-HA is a promising injectable scaffold with thermoresponsive properties suitable for in situ cartilage tissue engineering.
1. INTRODUCTION
Cartilage tissue engineering using autologous chondrocytes (or mesenchymal stem/stromal cells (MSCs) as a cell source) has been developed, and has been used in clinical applications. In the original system of chondrocytes implantation, a cell suspension of autologous chondrocytes expanded in monolayer culture was transplanted into defective articular cartilage under a cover of periosteal flap [1-3]. This was a pioneering system, with great promise for cartilage repair, although it had some disadvantages. These disadvantages included leakage of cells from the transplanted site and uneven distribution of the transplanted cells in the site due to gravity [4]. In the improved second generation of chondrocyte implanttation systems, tissue-engineered cartilage was prepared using in vitro three-dimensional culture of atelocollagen gel scaffolds embedded with chondrocytes or mesenchymal cells [5-7]. A number of other biodegradable scaffolds have also been studied for cartilage tissue engineering. Most of these scaffolds are gel or sponge forms made of collagen, gelatin, hyaluronan, chondroitin sulfate, chitin/chitosan, alginate, poly(lactide-co-glycolide), polycaprolactone, and their derivatives or composites [8-13]; these systems still require either a periosteal flap or substitute for fascia to cover engineered cartilage.
Current studies focus on the use of novel biomaterials possessing injectable and in situ gelling characteristics; these properties will facilitate minimally invasive procedures under microscopic surgery for chondrocyte transplantation. Thermoresponsive polymer-grafted polysaccharides, which have both the characteristics of biological activity derived from polysaccharides and the selfassembling ability of thermoresponsive units such as poly(N-isopropyl-acrylamide) (PNIPAAm), are biomaterials that could fulfill this purpose [14-16]. In particular, PNIPAAm-grafed hyaluronan (PNIPAAm-g-HA) has the ability to induce ectopic chondrogenesis in cooperation with transplanted chondrocytes and growth factors in animal experiments [16]. The strategy of graft polymerization and coupling of PNIPAAm to biomaterials is being rapidly adopted in biomedical field. For example, the self-assembling property of functional biomaterials can be exploited to develop drug delivery carriers and injectable scaffolds for tissue engineering, as well as for cell sheet engineering [14-19], because the lower critical solution temperature (LCST) of PNIPAAm in aqueous solution (approximately 32˚C) is suitable for self-assembly in physiological conditions. However, in cases where the local body temperature at the target site is close to the LCST of PNIPAAm, in situ gelation of injected PNIPAAm-grafted materials may be compromised in the target tissue. Thus, tuning of the LCST is critical for optimal thermoresponsive biomaterial function in tissue engineering applications. Reduction of polymer LCST is facilitated by copolymerization with more hydrophobic monomers, such as N-tert-butylacrylamide (NtBAAm) [20,21]. Indeed, the theoretical LCST of a homopolymer composed of NtBAAm is approximately –5˚C.
In the present study, we modified an injectable scaffold (PNIPAAm-g-HA) that we had previously developed for in situ cartilage engineering [16]. This was achieved by adding 5 mol% of NtBAAm to generate poly (NIPAAm-co-NtBAAm)-grafted hyaluronan [P(NIPAAmco-NtBAAm)-g-HA] (Figure 1). Biocompatibility of this novel biomaterial was evaluated in terms of cytotoxicity (colony-forming assay) and acute systemic toxicity assessment. To investigate its potential as a scaffold for cartilage tissue engineering, cartilage-related gene expression in chondrocytes embedded in the gel was also studied in vitro.
2. MATERIALS AND METHODS
2.1. Chemicals
N-isopropylacrylamide (NIPAAm), N-tert-butylacrylamide (NtBAAm), 2-aminoethanethiol hydrochloride (AET-HCl), and 2,2’-azobis(isobutyronitrile) (AIBN) were purchased from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan). 1-Ethyl-3-(3-dimethylamino-propyl)-carbodiimide hydrochloride (EDAC) was purchased from DOJINDO Laboratories (Kumamoto, Japan). Hyaluronic acid sodium salt from rooster comb, and other high-purity-grade reagents for chemical synthesis were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan).
2.2. Preparation of Poly(N-Isopropylacrylamideco-N-tert-Butylacrylamide)-Grafted Hyaluronan [P(NIPAAm-co-NtBAAm)-g-HA]
Amino-terminated poly(NIPAAm-co-NtBAAm) [P(NIPAAm-co-NtBAAm)-NH2] was prepared by radical polymerization using AIBN as an initiator, as previously reported [16]. Briefly, NIPAAm/NtBAAm (5 mol% NtBAAm), AIBN and AET were dissolved in degassed DMF under argon-atmosphere, and the polymerization was conducted for 7 h at 70˚C. The product solution was dialyzed against ultrapure water by using spectra/Por 7 (MWCO: 8000) for 48 h and then lyophilized. Graftpolymerizations of P(NIPAAm-co-NtBAAm)-NH2 to hyaluronan (HA) were carried out using water-soluble carbodiimide agent in phosphate buffer. Hyaluronic acid sodium salt (100 mg) and P(NIPAAm-co-NtBAAm)- NH2 (500 mg) were dissolved in 0.05 M phosphate buffer (pH 6.0), and then, EDAC was added to the solution. The reaction was conducted at 24˚C with stirring for 48 h. To remove non-reacted P(NIPAAm-co-NtBAAm)- NH2, the reaction mixture was dialyzed against excess ultrapure water using spectra/Por 7 (MWCO: 25,000) for 48 h, lyophilized, and washed with acetone. The products were dried and stored at room temperature in a desiccator.
2.3. Low Critical Solution Temperature Measurements
The optical transmittance of polymers in deionized water was measured at 500 nm at temperatures ranging from 25˚C to 45˚C with a V-630BIO spectrophotometer (JASCO) to estimate the phase-transition temperature of the specimens. The low critical solution temperature (LCST) of the polymer solution was determined as previously reported [15,16].
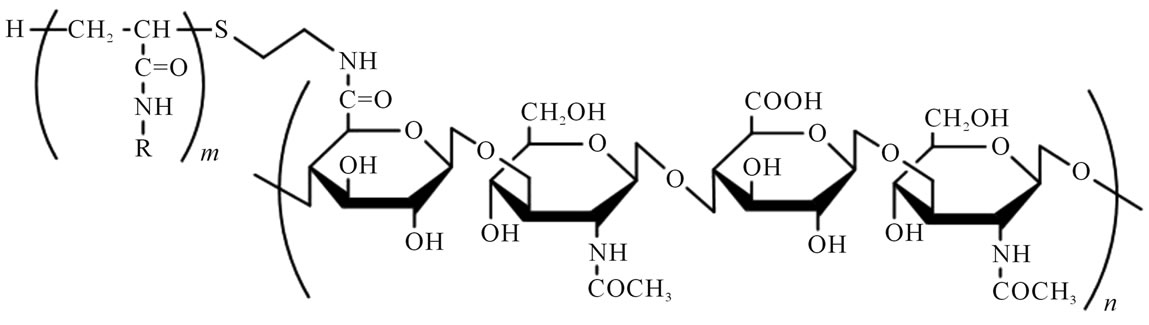
Figure 1. Chemical structure of poly(N-isopropylacrylamide-co-N-tert-butylacrylamide)-grafted hyaluronan [P(NIPAAm-co-NtBAAm)-g-HA]. R = CH(CH3)2 or C(CH3)3 (NIPAAm/NtBAAm molar ratio of 95/5).
2.4. Cytotoxicity Test (Colony-Forming Assay)
Cytotoxicity of the P(NIPAAm-co-NtBAAm)-g-HA was evaluated by means of the colony-forming assay using the Chinese hamster lung fibroblast cell line V79 (obtained from Health Science Research Resources Bank, Osaka, Japan). V79 cells suspended in Eagle’s minimum essential medium (MEM) containing 5% fetal bovine serum (FBS) were seeded into 12-well culture plates (50 cells/well) and incubated for 24 h in an atmosphere of 5% CO2 in air at 37˚C. After 24-h incubation, the medium of each well was changed individually to the sample-solubilized medium (at 0, 0.1, 0.5, 1.0, and 5 mg/mL final concentration). The cells were cultured for a week, then fixed with methanol, and stained with 2% Giemsa solution (Wako, Japan). Subsequently, the number of colonies consisting of 50 cells or more was counted with Model CL560 colony counter (Sibata Scientific technology Ltd., Saitama, Japan).
2.5. Acute Systemic Toxicity Assessment
All animal experiments in this study were approved by the Animal Care Committee at Tokyo Denki University. Jcl/ICR mice (age 4 weeks) were purchased from Clea Japan, Inc. (Tokyo, Japan), and kept under a condition with standard light-dark schedule, relative temperature, and humidity for 7 days to adapt to their environment. P(NIPAAm-co-NtBAAm)-g-HA was administered with saline at a dose of 2 mg/g body weight by intraperitoneal injection into mice (n = 5). Each mouse was clinically examined, and body weight was measured once a day for the initial 3 days and every other day for the following 4 days. After the mice were sacrificed and inspected for visible toxic damage, extracted organs, including the heart, lung(s), liver, spleen, and kidney(s), were weighed using an electronic balance AUX320 (Shimazu Co., Kyoto, Japan). Tissues were fixed with 10% formalin neutral buffer solution, dehydrated in ethanol and xylene, embedded in paraffin, and sliced onto aminosilanecoated glass. The specimens were stained by hematoxylin-eosin, and evaluated by microscopic examination using a DX51 microscope equipped with a DP71 digital camera system (Olympus, Tokyo, Japan).
2.6. Isolation and Three-Dimensional Cultures of Bone Marrow Derived Mesenchymal Cells
Rabbit bone marrow-derived mesenchymal stem/stromal cells (MSCs) were harvested from femur and tibia of New Zealand White male rabbits (age 6 - 8 weeks). Briefly, after anesthesia by intravenous injection of Somnopentyl (Kyoritsu Seiyaku Co., Tokyo, Japan), bone marrow fluid in each incised long bone was aspirated or flushed out using an 18-gauge needle and syringe (Terumo, Tokyo, Japan) containing 20 units of heparin. The aspirate was suspended in serum-free DMEM/F12 medium (Invitrogen) and passed through a 100-μm-mesh cell strainer, collected by centrifugation, and resuspended in fresh DMEM/F12 medium containing 10% FBS. After cells were cultivated in a monolayer, cells at passage 2 were used for in vitro three-dimensional culture. A mixture composed of 50 μL of 1.5 wt% P(NIPAAm-co-NtBAAm)- g-HA and 5 × 105 MSC cells were mixed in centrifuge microtubes to embed the cells into hydrogels, and then, warmed culture medium was added to the tube. Cells embedded in P(NIPAAm-co-NtBAAm)-g-HA hydrogels were cultivated in DMEM/F12 supplemented with 10% FBS, 10−7 M dexamethasone, 10 ng/mL of transforming growth factor (TGF-β1), and ITS (10 μg/mL insulin, 6.7 mg/mL transferring, and 5.5 μg/mL selenium) for 4 weeks at 37˚C with 5% CO2. As a standard for chondrogenic differentiation, a gel-free cell aggregate (5 × 105 cells) was also prepared by centrifugation as a positive control.
2.7. DNA Assay
At each time point of cultivation, cells were rinsed with PBS and homogenized in 10 mM Tri-HCl (pH 8.0) containing 1 mM EDTA and 1% Triton X-100 to analyze DNA contents. Double-stranded DNA was measured using bisbenzimide dye (Hoechst 33258) (Bio-Rad, Hercules, USA) according to the manufacturer’s instructions.
2.8. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from cells by using ISOGEN RNA isolation kit (Nippon Gene, Tokyo, Japan) with DNase I treatment according to the manufacturer’s instructions. One microgram of each RNA sample was reverse-transcribed into cDNA by using SuperScript II first-strand synthesis system for RT-PCR (Invitrogen). To analyze expression of specific genes, equal amounts of first strand cDNA were amplified by PCR using Ex Taq polymerase (Takara Bio Inc., Ohtsu, Japan) and specific forward and reverse primers for each gene (Table 1). The PCR conditions were as follows: 1 min at 94˚C, 30 sec at 55˚C, and 30 sec at 72˚C for 32 cycles. The products amplified by PCR were subjected to electrophoresis in a 2% agarose gel, and digital photographs of ethidium bromide-stained gels were taken with an ImageQuant LAS4000 image analyzer (GE Healthcare).
2.9. Statistical Analysis
Student’s unpaired t-test or the Aspin-Welch paired t-test was employed for equal variance or unequal variance,
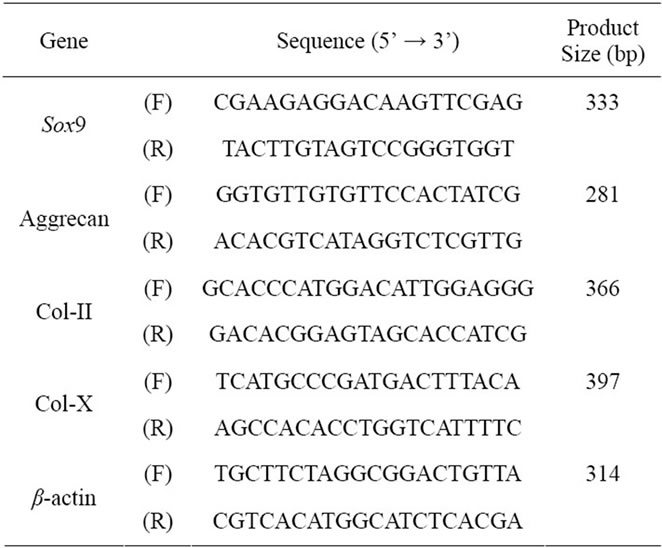
Table 1. Specific primers used in RT-PCR analysis.
respectively. Significant differences were determined at p < 0.05 for the t-test.
3. RESULTS
3.1. LCST of Synthesized P(NIPAAm-co-NtBAAm)-g-HA
The transmittance-temperature curve of the 0.5 wt% of thermoresponsive polymer-grafted HAs in aqueous solution is shown in Figure 2. The LCSTs of PNIPAAm-gHA and P(NIPAAm-co-NtBAAm)-g-HA were 32.0˚C and 28.6˚C, respectively (Table 2). These LCSTs were almost same as the LCST of each N-substituted acrylamide polymer itself.
3.2. Cytotoxicity Evaluation
The cytotoxicity of thermoresponsive-polymer-grafted HA derivatives was evaluated using a colony-forming assay. Colony-forming efficiency (CFE) of the HA derivatives is shown in Table 2. No cytotoxicity was detected over a range of concentrations up to 1 mg/mL, whereas a slight reduction in CEF was observed in all samples at the recommended maximum concentration (5 mg/mL). However, the values of 2 thermoresponsive polymer-grafted HA derivatives were higher than the value of unmodified hyaluronan.
3.3. Acute Systemic Toxicity Analysis
There were no obvious clinical signs related to the effects of intraperitoneal injection of P(NIPAAm-coNtBAAm)-g-HA in mice. Body-weight gain during the study did not differ between the control and sample injection group (Figure 3). The Summary of relative organ weights and histopathological findings of test animals are shown in Table 3; no significant differences were de-

Table 2. Characteristics of thermoresponsive polymer-grafted hyaluronan derivatives.
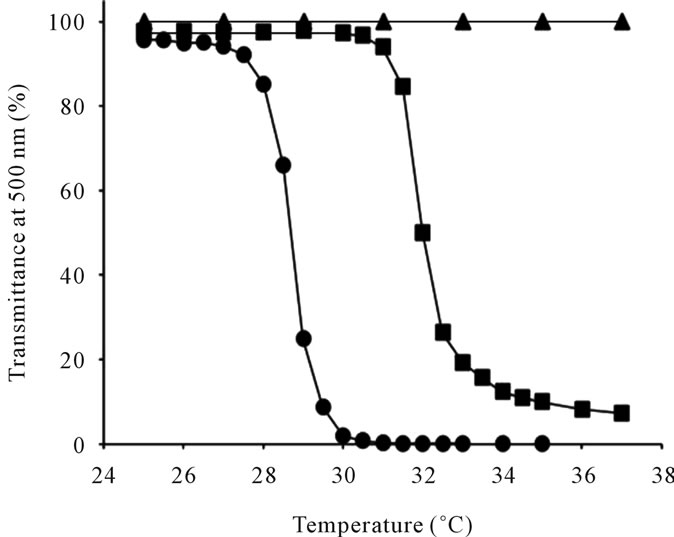
Figure 2. Transmittance as a function of temperature for (●) P(NIPAAm-co-NtBAAm)-g-HA with NIPAAm/NtBAAm molar ratio of 95/5, (■) PNIPAAm-g-HA, and (▲) hyaluronan (HA).
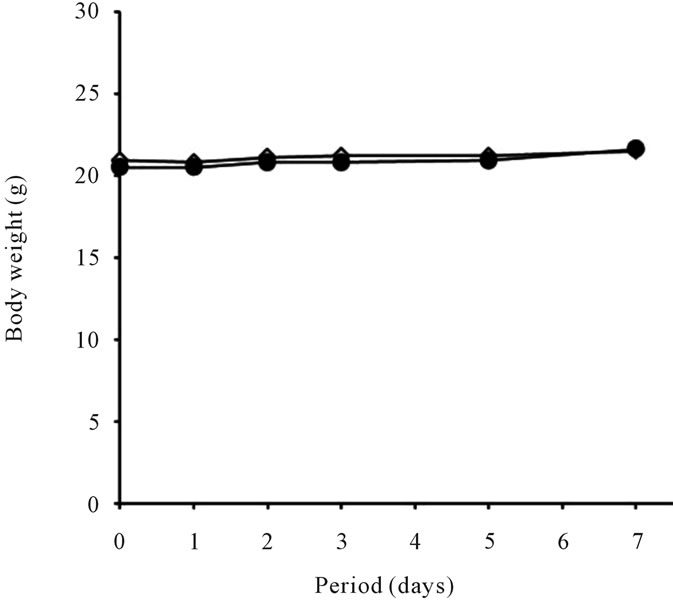
Figure 3. Body weight change of mice administrated P(NIPAAm-co-NtBAAm)-g-HA by intraperitoneal injection with saline at dosage of 2 mg/g body weight for a week. ●: P(NIPAAm-co-NtBAAm)-g-HA, △: control.
Table 3. Summary of relative organ weights and histopathological findings of mice injected with P(NIPAAm-co-NtBAAm)-g-HA.
tected between the groups. Figure 4 shows typical histological photographs of liver of mice 7 days after injection of P(NIPAAm-co-NtBAAm)-g-HA. We did not detected signs of inflammation or any other remarkable differences in comparison with normal tissue (control groups).
3.4. DNA Assay
Figure 5 shows the DNA content of MSCs in chondrogenesis at each time point. A transient increase in DNA content (indicative of proliferation) in response to TGF-β was observed in the P(NIPAAm-co-NtBAAm)-g-HA gel group at day 7, whereas no cell expansion was observed in gel-free aggregates at the same time point. Although DNA content at day 14 decreased relative to day 7 in both experimental groups, the initial amount was maintained for at least 14 days in the gel group only.
3.5. Gene Expression in the Engineered Cartilage Derived from MSCs
RT-PCR analysis using primers specific for cartilagerelated genes, including SRY-related HMG box-containing gene 9 (Sox9), gene encoding aggrecan, and genes encoding type-II and -X collagen, was conducted to characterize the phenotype of TGF-β-induced differentiation of MSCs to chondrocytes (Figure 6). The expression pattern of chondrocyte-specific genes in the P(NIPAAm-co-NtBAAm)-g-HA gel group was similar to that observed in gel-free aggregates. An exception was the Sox9 gene, which was more highly expressed in chondrogenic-differentiated MSCs embedded in the gel than in the aggregates.
4. DISCUSSION
The LCST of the original PNIPAAm-g-HA, which was developed as an injectable scaffold for in situ cartilage tissue engineering, is approximately 32˚C. This characteristic is theoretically compatible with in situ gelation at physiological temperatures [16]. However, efficient gelation of the material immediately after injection into
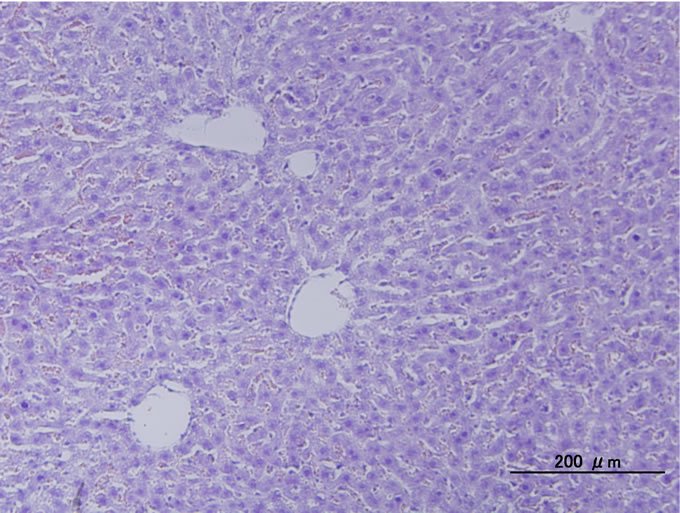
Figure 4. Typical histological photograph of liver of mice administrated P(NIPAAm-co-NtBAAm)-g-HA by intraperitoneal injection with saline at dosage of 2 mg/g body weight.
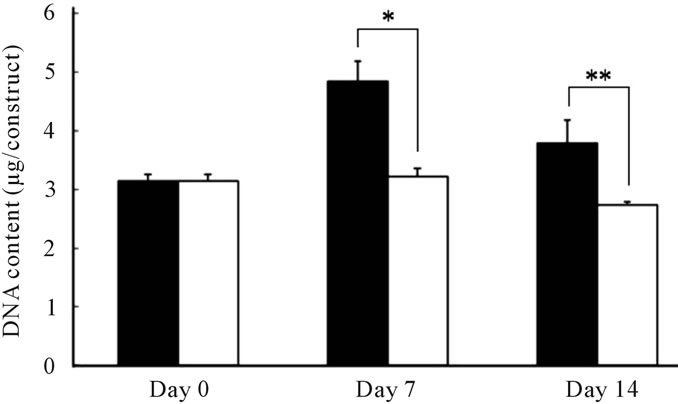
Figure 5. DNA content of MSCs differentiated to chondrocytes in (■) the P(NIPAAm-co-NtBAAm)-g-HA gel and (□) aggregates for 2 weeks. Statistically significant difference compared with control group at the level of *p < 0.01 or **p < 0.05.
knees deficient in cartilage is not always effective (private data). This is likely since body temperature around knee is close to the LCST of the material during the surgical procedure. Thus, we reasoned that reducing the LCST of PNIPAAm-g-HA may improve the gelation process. We achieved the reduction by copolymerization
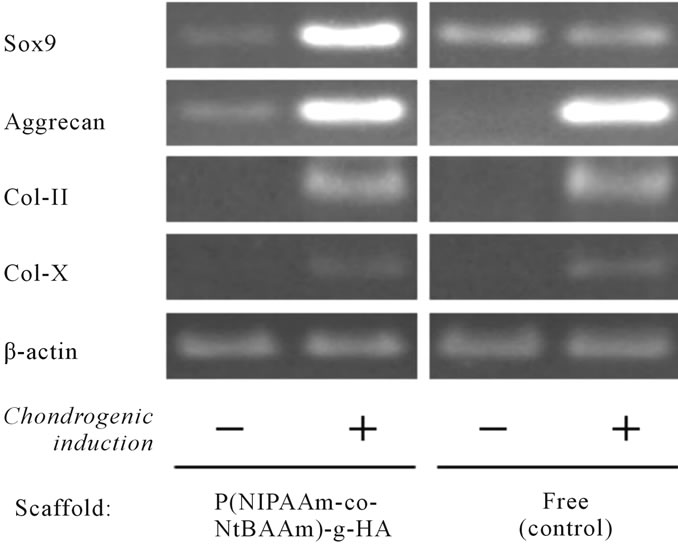
Figure 6. Expression of cartilage-related genes in MSC-derived chondrocytes embedded in P(NIPAAm-co-NtBAAm)-grafted hyaluronan at 4 weeks.
with N-tert-butylacrylamide (NtBAAm), a relative of PNIPAAm. The components were designed so that the LCST of P(NIPAAm-co-NtBAAm)-g-HA would be approximately 28˚C - 29˚C. This should confer the biomaterial with the characteristics of mobility under operation at room temperature and of complete self-assembly property just after implantation (Figure 2 and Table 2).
In addition to efficiency and functionality, one of the most important issues for novel biomaterials is biocompatibility. In the present study, cytotoxicity and acute systemic toxicity of P(NIPAAm-co-NtBAAm)-g-HA were first investigated. We used colony-forming assay for this study, as it is the most sensitive method for cytotoxicity assessment of biomaterials. Although the CFE of both thermoresponsive-hyaluronan derivative groups was reduced at the recommended maximum concentration (5 mg/mL), it was higher than that obtained with the same dose of unmodified hyaluronan. Thus, we infer that the cytotoxicity was not caused by the thermoresponsive polymer itself. Acute systemic toxicity assessment also confirmed the biological safety of novel P(NIPAAm-coNtBAAm)-g-HA as measured in terms of clinical signs, body-weight changes, organ weights, and histopathological findings (Figures 3-4 and Table 3). This experiment was designed in order to assess biological hazards up to 2000-fold over the maximum dosage likely to be used in clinical applications.
Because the bone marrow-derived MSCs are typical somatic stem cells with higher proliferating potency and multi-lineage potential [5,22-24], they are an alternative cell source for clinical bone and cartilage tissue engineering [25-27]. In cases of repair of large and fullthickness defects of articular cartilage, MSCs may be advantageous as a source of a large number of chondrogenic cells for transplantation. This represents an improvement over the use of biopsied chondrocytes from non-weight-bearing surfaces of the knee, because isolation and expansion of these cells are associated with fibrous dedifferentiation [28]. On the other hand, hyaluronan-based scaffolds support the differentiation of MSCs to chondrocytes [29-31], and high-molecular-weight hyaluronan could transduce biological signals to mesenchymal cells via hyaluronan receptors such as CD44 [32-34]. This study also confirmed that P(NIPAAm-co-NtBAAm)- g-HA, which is a novel hyaluronan-based thermoresponsive biomaterial, was compatible with MSCs in chondrogenesis. DNA content (which correlates with cell number) was greater in the P(NIPAAm-co-NtBAAm)-g-HA group than in the control aggregates over an initial 2-week periods (Figure 5). The reason for this observation may be that the gel provides a penetrable network that offers sufficient space for initial cell proliferation and migration without excess cell-cell contact, whereas cells in the aggregate are restricted by their neighbors. However, cell number did eventually decrease after 1 week in culture during differentiation in both groups of the present study. This finding is consistent with that of other studies showing similar declines in the number of MSC-derived chondrocytes in three-dimensional scaffold cultures [35,36]. At 4 weeks, when sufficient numbers of chondrogenic cells were derived from MSCs, P(NIPAAm-co-NtBAAm)-gHA did not inhibit chondrogenic differentiation. Furthermore, Sox9 expression in the gel group was higher than in the control group at this time point (Figure 6). These findings indicate that the novel P(NIPAAm-co-NtBAAm)- g-HA biomaterial is more useful as an injectable scaffold for cartilage tissue engineering using MSCs than the original PNIPAAm-g-HA, because its reduced LCST renders it amenable for self-assembly in the body.
5. CONCLUSION
In the present study, we successfully prepared a novel biomaterial, P(NIPAAm-co-NtBAAm)-g-HA, that completely self-assembles at temperatures lower than those of previous compounds. No cytotoxicity or acute systemic toxicity was associated with this thermoresponsive HA derivative. P(NIPAAm-co-NtBAAm)-g-HA was also compatible with the production of MSC-derived chondrocytes. Taken together, although further study is necessary, P(NIPAAm-co-NtBAAm)-g-HA is a promising efficient biomaterial for in situ cartilage engineering, because it has biocompatible, injectable, and desirable thermo-dependent self-assembling properties.
6. ACKNOWLEDGEMENTS
The authors thank Miss Nana Hiura, Mr. Kousaku Toyoda, Miss Erika Ariyama for technical assistances. This study was partially supported by a grant of the Strategic Research Foundation Grant-aided Project for Private Universities from the Ministry of Education, Culture, Sport, Science, and Technology, Japan (MEXT), 2008-2012 (S0801023).
![]()
![]()
REFERENCES
- Grande, D.A., Pitman, M.I., Peterson, L., Menche, D. and Klein, M. (1989) The repair of experimentally produced defects in rabbit articular cartilage by autologous chondrocyte transplantation. Journal of Orthopaedic Research, 7, 208-218. doi:10.1002/jor.1100070208
- Brittberg, M., Lindahl, A., Nilsson, A., Ohlsson, C., Isaksson, O. and Peterson, L. (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New England Journal of Medicine, 331, 889-895. doi:10.1056/NEJM199410063311401
- Peterson, L., Minas, T., Brittberg, M., Nilsson, A., SjogrenJansson, E. and Lindahl, A. (2000) Twoto 9-year outcome after autologous chondrocytes transplantation of the knee. Clinical Orthopaedics and Related Research, 374, 412-434. doi:10.1097/00003086-200005000-00020
- Sohn, D.H., Lottman, L.H., Lum, L.Y., Kim, S.G., Pedowitz, R.A., Coutts R.D. and Sah, R.L. (2002) Effect of gravity on location of chondrocytes implanted in cartilage defects. Clinical Orthopaedics and Related Research, 394, 254-262. doi:10.1097/00003086-200201000-00030
- Wakitani, S., Goto, T., Pineda, S.J., Young, R.G., Mansour, J.M., Caplan, A.I. and Goldberg, V.M. (1994) Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. The Journal of Bone and Joint Surgery, 76, 579-592.
- Kawamura, S., Wakitani, S., Kimura, T., Maeda, A., Caplan, A.I., Shino, K. and Ochi, T. (1998) Articular cartilage repair: Rabbit experiments with a collagen gelbiomatrix and chondrocytes cultured in it. Acta Orthopaedica Scandinavica, 69, 56-62. doi:10.3109/17453679809002358
- Ochi, M., Uchio, Y., Kawasaki, K., Wakitani, S. and Iwasa, J. (2002) Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. Journal of Bone and Joint Surgery [Br.], 84, 571-578. doi:10.1302/0301-620X.84B4.11947
- Britt, J.C. and Park, S.S. (1998) Autogenous tissue-engineered cartilage: Evaluation as an implant material. Archives of Otolarygology—Head & Neck Surgery, 124, 671-677.
- Solchaga, L.A., Dennis, J.E., Goldberg, V.M. and Caplan, A.I. (1999) Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. Journal of Orthopaedic Research, 17, 205-213. doi:10.1002/jor.1100170209
- Gugala, Z. and Gogolewski, S. (2000) In vitro growth and activity of primary chondrocytes on a resorbable polylactide-dimensional scaffold. Journal of Biomedical Materials Research, 49, 183-191. doi:10.1002/(SICI)1097-4636(200002)49:2<183::AID-JBM5>3.0.CO;2-D
- Ponticiello, M.S., Schinagl, R.M., Kadiyara, S. and Barry, F.P. (2000) Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. Journal of Biomedical Materials and Research, 52, 246-255. doi:10.1002/1097-4636(200011)52:2<246::AID-JBM2>3.0.CO;2-W
- Ma, H.-L., Hung, S.-C., Lin, S.-Y., Chen, Y.-L. and Lo, W.-H. (2003) Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. Journal of Biomedical Materials Research, 64A, 273-281. doi:10.1002/jbm.a.10370
- Abe, M., Takahashi, M., Tokura, S., Tamura, H. and Nagano, A. (2004) Cartilage-scaffold composites produced by bioresorbable β-chitin sponge with cultured rabbit chondrocytes. Tissue Engineering, 10, 585-594. doi:10.1089/107632704323061942
- Cho, J.H., Kim, S.-H., Park, K.D., Jung, M.C., Yang, W.I., Han, S.W., Noh, J.Y. and Lee, J.W. (2004) Chondrogenic differentiation of human mesenchymal stem cells using a thermosensitive poly(N-isopropylacrylamide) and watersoluble chitosan copolymer. Biomaterials, 25, 5743-5751. doi:10.1016/j.biomaterials.2004.01.051
- Chen, J.-P. and Cheng, T.-H. (2006) Thermo-responsive chitosan-graft-poly(N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromolecular Bioscience, 6, 1026-1039. doi:10.1002/mabi.200600142
- Muramatsu, K., Ide, M. and Miyawaki, F. (2012) Biological evaluation of tissue-engineered cartilage using thermoresponsive poly(N-isopropylacrylamide)-grafted hyaluronan. Journal of Biomaterials and Nanobiotechnology, 3, 1-9. doi:10.4236/jbnb.2012.31001
- Uludag, H., Norrie, B., Kousinioris, N. and Gao, T.J. (2001) Engineering temperature-sensitive poly(N-isopropylacrylamide) polymers as carriers of therapeutic proteins. Biotechnology and Bioengineering, 73, 510-521. doi:10.1002/bit.1086
- Kim, J.-H., Kim, Y.-S., Park, K., Kang, E., Lee, S., Nam, H.Y., Kim, K., Park, J.H. Chi, D.Y., Park, R.-W., Kim, I.-S., Choi, K. and Kwon, I.C. (2008) Self-assembled glycol chitosan nanoparticles for the sustained and prolonged delivery of antiangiogenic small peptide drugs in cancer therapy. Biomaterials, 29, 1920-1930. doi:10.1016/j.biomaterials.2007.12.038
- Yamada, N., Okano, T., Sakai, H., Karikusa, F., Sawasaki, Y. and Sakurai, Y. (1990) Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Macromolecular Chemistry, Rapid Communication, 11, 571-576. doi:10.1002/marc.1990.030111109
- Liu, H.Y. and Zhu, X.X. (1999) Lower critical solution temperatures of N-substituted acrylamide copolymer in aqueous solutions. Polymer, 40, 6985-6990. doi:10.1016/S0032-3861(98)00858-1
- Hoffman, A.S., Stayton, P.S., Bulmus, V., Chen, G., Chen, J., Cheung, C., Chilkoti, A., Ding, Z., Dong, L., Fong, R., Lackey, C.A., Long, C.J., Miura, M., Morris, J.E., Murthy, N., Nabeshima, Y., Park, T.G., Press, O.W., Shimoboji, T., Shoemaker, S., Yang, H.J., Monji, N., Nowinski, R.C., Cole, C.A., Priest, J.H., Harris, J.M., Nakamae, K., Nishino, T. and Miyata, T. (2000) Really smart bioconjugates of smart polymers and receptor proteins. Journal of Biomedical Materials Research, 52, 577-586. doi:10.1002/1097-4636(20001215)52:4<577::AID-JBM1>3.0.CO;2-5
- Wakitani, S., Saito, T. and Caplan, A.I. (1995) Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle & Nerve, 18, 1417- 1426. doi:10.1002/mus.880181212
- Pittenger, M.F., Mackey, A.M., Beck, S.C., Jaiswal, R.K., Douglas, R., Mosca, J.D., Moorman, M.A., Simonetti, D.W., Craig, S. and Marshak, D.R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science, 284, 143-147. doi:10.1126/science.284.5411.143
- Woodbury, D., Schwarz, E.J., Prockop, D.J. and Black, I.B. (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. Journal of Neuroscience Research, 61, 364-370. doi:10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C
- Ohgushi, H., Kitamura S., Kotobuki, N., Hirose, M., Machida, H., Muraki, K. and Takakura, Y. (2004) Clinical application of marrow mesenchymal stem cells for hard tissue repair. Yonsei Medical Journal, 45, 61-67.
- Kuroda, R., Ishida, K., Matsumoto, T., Akusue, T., Fujioka, H., Mizuno, K., Ohgushi, H., Wakitani, S. and Kurosaka, M. (2007) Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis and Cartilage, 15, 226-231. doi:10.1016/j.joca.2006.08.008
- Nejadnik, H., Hui, J.H., Choong, E.P.F., Tai, B.C. and Lee, E.H. (2010) Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. The American Journal of Sports Medicine, 38, 1110-1116. doi:10.1177/0363546509359067
- Grundmann, K., Zimmermann, B., Barrach, H.-J. and Merker, H.-J. (1980) Behavior of epiphyseal mouse chondrocyte populations in monolayer culture, Morphological and immunohistochemical studies. Virchows Archiv A: Pathological Anatomy and Histology, 389, 167-187. doi:10.1007/BF00439484
- Angele, P., Kujat, R., Nerlich, M., Yoo, J., Goldberg, V. and Johnstone, B. (1999) Engineering of osteochondral tissue with bone marrow mesenchymal progenitor cells in a derivatized hyaluronan-gelatin composite sponge. Tissue Engineering, 5, 545-554. doi:10.1089/ten.1999.5.545
- Radice, M., Brun, P., Cortivo, R., Scapinelli, R., Battaliard, C. and Abatangelo, G. (2000) Hyaluronan-based biopolymers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. Journal of Biomedical Materials Research, 50, 101-109. doi:10.1002/(SICI)1097-4636(200005)50:2<101::AID-JBM2>3.0.CO;2-M
- Lisignoli, G., Cristino, S., Piasentini, A., Cavallo, C., Caplan, A.I. and Facchini, A. (2006) Hyaluronan-based polymer scaffold modulates the expression of inflammatory and degradative factors in mesenchymal stem cells: Involvement of Cd44 and Cd54. Journal of Cellular Physiology, 207, 364-373. doi:10.1002/jcp.20572
- Knudson, C.B. (2003) Hyaluronan and CD44: Strategic players for cell-matrix interactions during chondrogenesis and matrix assembly. Birth Defects Research Part C: Embryo Today: Reviews, 69, 174-196. doi:10.1002/bdrc.10013
- Yatabe, T., Mochizuki, S., Takizawa, M., Chijiiwa, M., Okada, A., Kimura, T., Fujita, H., Toyama, Y. and Okada, Y. (2009) Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Annals of the Rheumatic Diseases, 68, 1051-1058. doi:10.1136/ard.2007.086884
- Julovi, S.M., Ito, H., Hishitani, K., Jackson, C.J. and Nakamura, T. (2010) Hyaluronan inhibits matrix metalloproteinase-13 in human arthritic chondrocytes via CD44 and P38. Journal of Orthopaedic Research, 29, 258-264. doi:10.1002/jor.21216
- Park, H., Temenoff, J.S., Tabata, Y., Caplan, A.I. and Mikos, A.G. (2007) Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials, 28, 3217-3227. doi:10.1016/j.biomaterials.2007.03.030
- Liao, J., Guo, X., Allen, K.J.G., Kasper, F.K. and Mikos, A.G. (2010) Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials, 31, 8911-8920. doi:10.1016/j.biomaterials.2010.07.110

