 Food and Nutrition Sciences, 2013, 4, 14-27 http://dx.doi.org/10.4236/fns.2013.47A003 Published Online July 2013 (http://www.scirp.org/journal/fns) Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats Young Chul Lee1, Eujin Hyun1, Mesfin Yimam2, Lidia Brownell2, Qi Jia2 1Unigen Inc., Cheonan, South Korea; 2Unigen Inc., Seattle, USA. Email: yclee@unigen.net, QJia@unigen.net Received March 21st, 2013; revised April 22nd, 2013; accepted April 30th, 2013 Copyright © 2013 Young Chul Lee et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT UP446 has been used in both joint supplements and prescription medical food. The purpose of this study was to evalu- ate the pharmaceutical safety of UP446 via acute and 26-week repeated oral dose toxicity study in SD rats. In acute tox- icity study, UP446 was administered by oral gavage to Sprague-Dawley rats (5 males and 5 females) at a dose of 5000 mg/kg. In 26-week repeated oral dose toxicity study, UP446 at doses of 500, 1000 and 2000 mg/kg/day were given orally to groups of rats (10 rats/dose/sex) for 26-week. UP446 at a dose of 5000 mg/kg produced no treatment-related acute toxicity or mortality in any of the animals tested during 14 days of the study. In 26-week repeated dose toxicity study, there was no significant difference in body weight between the control and all treatment groups. Blackish stool and soft stool was observed in one male in the 1000 mg/kg group and in some males and females of 2000 mg/kg group. However, these changes of stool were not considered to be toxic effects because neither histopathological change in gastrointestinal tracks (GIT) nor body weight change were detected. No drug induced abnormalities were found as of body weights, food consumption, ophthalmological examinations, urinalysis, hematology, clinical chemistry, organ weights and gross necropsy in any animals in the dosing groups. These results suggest that the oral lethal dose of UP446 for male and female rats is in excess of 5000 mg/kg and the no observed adverse effect level (NOAEL) of the UP446 for both male and female rats is considered to be greater than 2000 mg/kg/day. Keywords: Scutellaria baicalensis; Acacia catechu; Acute Oral Toxicity; Repeated Oral Toxicity 1. Introduction The formulations containing multiple plant extracts have attained wide recognition in comparison to crude plant materials and extracts, due to reduction in dose, conven- ience, and ease of administration. These formulations are used by large sections of the human population particu- larly in developed countries. Many synthetic drugs are known to act on a single molecular target but the multi- target responses of herbal formulations are proven to be beneficial in chronic conditions [1-3]. The World Health Organization (WHO) insists that the safety of herbal medicines is a critical component in the quality control of healthcare products. However, lack of adequate regula- tions, the pharmacological complexity of herbal products, and the insufficiency of information on the pharmacol- ogy and toxicity of these compounds are sometimes be- coming medical issues, even though the side effects are minimal [4,5]. Thus a crucial standardization and toxicological evalu- ation of the safety in pre-clinical model could be relevant. Flavonoids are polyphenolic compounds that are ubiqui- tous in nature and are categorized, according to chemical structure, into flavonols, flavones, flavanones, isoflavones, catechins, anthocyanidins, and chalcones. Over 4000 flavonoids have been identified, many of which present in daily consumed human foods such as fruits, vegetables, and beverages including tea, coffee, beer, wine, and fruit drinks as well as traditional medicines and pharmaceuti- cal drugs [6]. Usage of flavonoids include food flavor, fruits and vegetables pigments, antimicrobial, antiviral, antioxidants [7-9], as well as anticarcinogenic and an- timutagenic [10]. Primarily two bioflavonoids, baicalin from Scutellaria baicalensis (S. baicalensis) and catechin from Acacia catechu (A. catechu) have been used sepa- rately in many traditional medicines and pharmaceutical products for a variety of uses including anti-inflamma- Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 15 tory, antiviral, antibacterial, anticancer, and cardiovascu- lar applications [7,11-17]. UP446 is a standardized bioflavonoid composition with primarily baicalin from the roots of Scutellaria bai- calensis (S. baicalensis) and (+)-catechin from the heart- woods of Acacia catechu (A. catechu) [18]. It has been used in both over the counter joint care dietary supple- ments and prescription medical food [19]. The therapeu- tic dosage of UP446 is 250 - 500 mg per day in clinical applications [20]. Previous studies have shown that UP446 reduces production of eicosanoids through inhibition of cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2), and 5-lipoxygenase (5-LOX) enzymes and also decreases expressions of inducible nitric oxide synthase (iNOS), nuclear factor-kappaB (NF-κB), and tumor necrosis fac- tor-alpha (TNF-α) [21]. Furthermore, UP446 showed anal- gesic effects on multiple in vivo models [22]. The safety of UP446 was partially evaluated in 90 days repeated oral toxicity study in rats [23] but long term assessment for toxicity in rats is required because most patients with chronic arthritis diseases undergo long term therapy. The objective of the current study is to provide a com- prehensive supplement to the safety profile reported pre- viously for a standardized composition of S. baicalensis and A. catechu. Herein, two toxicity studies were per- formed to evaluate the safety of UP446. The acute oral toxicity study was carried out at a high dose, whereas the 26-week repeated oral toxicity study was performed to establish the no-observed-adverse-effect level (NOAEL) of UP446. 2. Materials and Methods 2.1. Preparation of UP446 Detailed method for preparation of the two major flavon- oids, baicalin and catechin, from the roots of S. baicalen- sis and the heartwoods of A. catechu, respectively, were disclosed in a US patent [24]. Briefly, S. baicalensis ex- tract from roots was extracted with water and then re- crystallized. The S. baicalensis extract contained baicalin as the major bioflavonoid at content not less than 75% as well as other minor free-B-ring flavonoids such as wo- gonin-7-O-G-glucuronide, oroxylin A-7-OG-glucuronide, and baicalein. Catechin extract was obtained from re- peated crystallization of an aqueous extraction of the heartwoods of an India medicinal plant, A. catechu. (+)- Catechin is the major component in the A. catechu ex- tract with a content of not less than 65% plus a minor amount of its enantiomer, epicatechin, as well as other minor amounts of flavans. Analyses of the extracts were performed separately by two high-performance liquid chromatography (HPLC) methods. The quantification re- sults of baicalin, from the S. baicalensis extract and catechin from the A. catechu extract were calculated by comparison HPLC peak area with known standards. The final UP446 composition was a mixture of S. baicalensis and A. catechu standardized extracts at a ratio 4:1 with baicalin content not less than 60% and catechin content not less than 10%. Other minor flavonoids, such as wo- gonin 7-glucuronide and baicalein, account for about 15% of total weight. Moisture, ash, fat, and fiber constitute the remainder weight (Figure 1). 2.2. Experimental Animals Male and female Sprague–Dawley rats, aged 5 weeks, were purchased from the ORIENTBIO INC. in Korea. In the acute oral toxicity study, 10 rats of each sex were used, whereas in the 26-week repeated oral dose toxicity study, 50 rats of each sex including recovery groups were used. All rats were labeled by tailmark and housed singly in stainless wire mesh cages in a room maintained under environmentally controlled conditions of 21˚C ± 2˚C and a 12 h light-dark cycle in the animal facility of Biotox- tech Co., Ltd. All rats were acclimatized at least one week before starting the experiments, and had free ac- cess to water and food. Test article was mixed in a solu- tion of 0.5% carboxymethylcellulose sodium salt (CMC- Na) in water (Choongwae Pharma Corp). The control group received the vehicle only at the same volume as the test animals. Clinical signs were observed in all ani- mals during the experiments. 2.3. Acute Oral Toxicity Study This study was performed at the Biotoxtech GLP insti- tute following approval of the Institutional Animal Care and Use Committee (Approval No.: 110947 and Study No.: B11996) based on Animal Protection Act and con- ducted in compliance with the Organization for Eco- nomic Cooperation and Development (OECD) Guideline 425 adopted on the 3rd of October 2008 and Korea Food and Drug Administration (KFDA) Guideline. After acclimatization, 10 rats of each sex were ran- domly divided into two groups of 5 males and 5 females and were treated by gavage at doses of 0 and 5000 mg/kg body weight. Mortality, clinical signs, body weight chan- ges and gross findings were monitored during the 14 days after treatment. 2.4. 26-Week Repeated Oral Dose Toxicity Study This study was performed at the Biotoxtech GLP insti- tute following approval of the Institutional Animal Care and Use Committee (Approval No.: 110736 and Study No.: B11287) and conducted in compliance with the Or- anization for Economic Cooperation and Development g Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats Copyright © 2013 SciRes. FNS 16 Minutes 05 10 15 20 25 30 mAU 0 500 1000 1500 0 500 1000 1500 Baicalin (a) Minutes 024681012 mAU 0 200 400 600 0 200 400 600 Catechin Epicatechin (b) Figure 1. HPLC chromatogram of UP446. (a) Baicalin contents in UP446; (b) Catechin contents in UP446. (OECD) Guideline, ICH Harmonised Tripartite Guide- line and Korea Food and Drug Administration (KFDA) Guideline. 40 male and 40 female rats were divided into four groups of 10 animals each; 10 additional animals of each sex were used as recovery groups: five for the con- trol and the other five for the high-dose group. UP446 (500, 1000, or 2000 mg/kg) or control group was orally administered once daily using an oral Zonde needle for 6 months. Mortality, clinical signs, body weight, food con- sumption and gross findings were monitored during ex- perimental period. Ophthalmoscopic examinations were conducted on both eyes of 5 animals/sex/group in the main group and those of all animals in the recovery group at 26 weeks and 30 weeks. At the end of the study, all surviving animals were fasted overnight. 3 hours and 24 hours urine samples were collected from 5 animals/ sex/group in the main group and all animals in the re- covery group prior to blood collection. Animals were anesthetized with isoflurane and blood samples were col- lected from the abdominal aorta and used to measure hematological and biochemical parameters. The hemato- logical parameters assessed included erythrocyte count (RBC), hemoglobin (HGB), Hematocrit (HCT), Mean corpuscular volume (MCV), Mean corpuscular hemoglo- bin (MCH), Mean corpuscular hemoglobin concentration (MCHC), platelets (PLT), leukocyte count (WBC), WBC differential counting, Reticulocyte (Reti), prothrombin time (PT) and activated partial thromboplastin time (APTT). The clinical chemistry parameters assessed in- cluded alanine aminotransferase (ALT), aspartate ami- notransferase (AST), alkaline phosphatase (ALP), gam- ma glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), creatinine (Crea), total bilirubin (T-Bili), total protein (TP), albumin (Alb), Total cholesterol (T-Chol), triglycerides (TG) and glucose (Glu). After blood collec- tion, all animals were immediately sacrificed for gross pathological examination of the internal organs. The or- gans such as brain, pituitary, thymus, heart, lung, liver, spleen, kidney, adrenal and sex organs were removed, blotted free of blood and weighed immediately. Histopa- thological examinations of animals were performed in the control group and the highest dose group, and in the  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 17 lower dose groups when appropriate. All organs were fixed in 10% neutral-buffered formalin, and the tissue sections were prepared with hematoxylin and eosin stain- ing before microscopic examination for pathological chan- ges. 2.5. Statistical Analysis Statistical analysis was performed using SAS program (version 9.2, SAS Institute Inc., USA). Body weights in acute oral toxicity study were analyzed utilizing Folded- F test for homogeneity of variance. And then Student t- test was employed on homogeneous data for confirming significance. Body weight, food consumption, urine vol- ume, hematology, clinical chemistry and organ weight data in 26-week repeated oral dose toxicity study were analyzed utilizing Bartlett’s test for homogeneity of va- riance. One-way analysis of variance (ANOVA) was em- ployed on homogeneous data; then, if significant, Dun- nett’s test was applied for multiple comparisons. Krus- kal-Wallis test was employed on heterogeneous data. The p values less than 0.05 were considered significant. 3. Results 3.1. Acute Oral Toxicity Study To evaluate the acute oral toxicity of UP446, both sexes of rats were orally administrated UP446 at the dose of 5000 mg/kg body weight. The UP446 at a dose of 5000 mg/kg produced no treatment related toxicity or mortality in any of the animals during 14 days of the study. In ad- dition, no body weight loss was detected (Table 1) and all internal organs examined at necropsy were free from any gross pathological changes. Therefore, the results suggest that the lethal dose of UP446 is greater than 5000 mg/kg in male and female rats. 3.2. 26-Week Repeated Oral Dose Toxicity Study No deaths and no treatment-related signs of toxicity were observed throughout 26 and 30 weeks of the study in any of the groups except one male and female in the control group. Appearance and behavior of the animals were similar for all groups of animals. There was no statistically significant difference in body weight between the control and all treatment groups (Figure 2). Blackish stool was observed in males and females in the 500, 1000 and 2000 mg/kg UP446 groups. Soft stool was observed in one male in the 1000 mg/kg group and in some males and females in the 2000 mg/kg group (Table 2). However, these were not considered to be toxic effects because neither histopathological change in gastrointestinal tracks (GIT) nor body weight change was detected. There were no associations between treat- ment and the findings recorded in the ophtalmological examinations conducted at the end of the treatment and recovery periods (Tab le s 3 and 4). Urinalysis results also showed that no test substance related changes in males and females in the dosing groups in the main group and in the recovery group (data not shown). Some hemato- logical parameters of female rats treated with UP446 at the dose of 2000 mg/kg/day showed statistically signifi- cant differences when compared to those of the control group. After 26-week of treatment, female rats had a slightly decreased RBC, HGB and HCT at 2000 mg/kg. However, these effects were not observed in recovery group and male group (Tables 5 and 6). There were no test substance related changes in males and females in the dosing groups in the main group and in the 2000 mg/kg group in the recovery group. Serum clinical chemistry data were shown in Ta bles 7 and 8. In male, a statistically significant decreased in glucose at 2000 mg/kg when compared with that of the control group. There were no significant differences be- tween treatment and control groups in females. The absolute and relative internal organ weights of male and female rats treated with UP446 are summarized in Tables 9 and 10. In absolute and relative organ weights, no statistically significant changes were shown in the treatment group when compared to control group. However, the absolute adrenal gland weight was signifi- Table 1. Body weight and body weight gain on da y 14 of rats in the acute oral toxicity study. Body weight (g) Gain (g) Group Day 0 Day 1 Day 3 Day 7 Day 14 Day 0-14 Male Control 162.9 ± 2.9 189.0 ± 4.3 212.6 ± 3.4 250.5 ± 6.6 314.0 ± 10.8 151.1 ± 8.7 UP446, 5 g/kg 163.1 ± 4.9 188.6 ± 4.8 211.2 ± 6.1 251.6 ± 8.7 317.4 ± 16.7 154.4 ± 13.9 Feamle Control 130.9 ± 2.9 147.6 ± 4.7 162.0 ± 4.7 177.7 ± 6.0 207.3 ± 14.1 76.4 ± 11.0 UP446, 5 g/kg 131.1 ± 2.9 145.7 ± 1.7 163.5 ± 6.4 180.3 ± 8.9 203.0 ± 18.1 71.9 ± 17.7 V alues are means ± SD (n = 5). Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 18 Table 2. Clinical signs of UP446 for 26-week repeated oral dose toxicity in male and female. No. of animals Clinical signs No. of animals affected Male Control Decrease in food intake 1 Decrease of fecal volume 1 Prone position 1 Death 1 UP446, 500 mg/kg 10 Blackish stool 10 UP446, 1000 mg/kg 10 Blackish stool 10 Soft stool 1 Loss of teeth (upper teeth)1 UP446, 2000 mg/kg 15 Blackish stool 15 Soft stool 3 Female Control 15 Prone position 1 Death 1 UP446, 500 mg/kg 10 Blackish stool 15 Wound (anterior neck) 1 Crust formation (anterior neck) 1 UP446, 1000 mg/kg 10 Blackish stool 10 UP446, 2000 mg/kg 15 Blackish stool 15 Soft stool 4 cantly increased in female recovery group only. That or- gan did not show any histopathological changes and just exhibit in female recovery group only. There were no test substance-related changes in male and females in the dosing groups in the main group and in the 2000 mg/kg group in the recovery group. The gross and histopathological examination revealed no findings related to the treatment (data not shown). The findings were observed in a few animals or were equally distributed among the groups, including in the control. They were considered spontaneous changes that occur commonly in normal rats of this strain and age, unrelated to the administration of UP446. 4. Discussion The present study demonstrated the comprehensive safety profile of standardized composition of bioflavon- oids extracted from S. baicalensis and A. catechu at a dose as high as 5000 mg/kg in the acute toxicity study and 2000 mg/kg in the 26-week repeated oral admini- stration of. Previous study confirmed the safety of UP446 (a) (b) Figure 2. Effects of oral administration of UP446 for 26- week repeated oral dose toxicity with 4-week recovery pe- riod on mean body weights. (a) Body weight of male group (G1: control; G2: UP446 500 mg/kg; G3: UP446 1000 mg/kg; G4: 2000 mg/kg); (b) Body weight of female group (G1: control; G2: UP446 500 mg/kg; G3: UP446 1000 mg/kg; G4: 2000 mg/kg). Data expressed as mean ± SD. in 3 months repeated toxicity study using rats. NOAEL was assumed to be over 1000 mg/kg/day [23]. However, the systemic toxic effects of acute and long term treat- ment should be evaluated since the target diseases of UP446 are chronic arthritis that required long term treat- ment. In the acute toxicity study, UP446 caused neither treat- ment related signs of toxicity nor mortality during 14 days of the study. No test substance related effects on body weight and necropsy were observed in any animals at 5000 mg/kg group. Therefore, the lethal dose of UP446 is greater than 5000 mg/kg in male and female rats. In 26-week repeated oral dose toxicity study, no deaths and no treatment related signs were observed in animals of all groups except one male and female of control group. Food consumption of UP446 treated groups were found to be insignificant in male when compared to the control groups but significantly increased food con- sumption was observed in females in the 2000 mg/kg group at week 14 when compared to the control group (data not shown). However, these changes in food con- sumption were not considered to be test substance related effects since these were observed incidentally. Blackish stool and soft stool was observed in one male in the 1000 Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 19 Table 3. Urinalysis results of UP446 for 26-week repeated oral dose toxicity with 4-week recover y period in male rats. Parameters Grade Main group (mg/kg) Recovery group (mg/kg) Group/dose (mg/kg) Control UP446, 500UP446, 1000UP446, 2000Control UP446, 2000 No. of animals 5 5 5 5 5 5 Volume (mL) 14.4 ± 6.7 17.0 ± 3.9 18.8 ± 8.3 12.2 ± 3.0 9.4 ± 2.4 8.3 ± 2.9 Color Pale yellow 4 Yellow 1 5 5 5 5 5 Transparency Clear 5 5 5 5 5 5 Mild turbidity Turbidity pH 5 6 6.5 1 2 7 2 3 2 3 2 8 2 1 3 4 9 1 1 3 Protein (mg/dL) 3 5 2 4 1 25 2 2 1 4 3 75 1 1 150 1 500 Glucose (mg/dL) Normal 5 5 5 5 5 5 50 100 300 1000 Ketone body (mg/dL) 5 5 5 5 5 5 5 15 50 100 Bilirubin (mg/dL) 5 5 5 5 5 5 1 3 4 Occult blood (Ery/μL) 5 5 5 5 4 5 10 1 25 50 150 Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 20 Continued 250 Cast‡ 0 5 5 5 5 5 5 1 - 5 6 - 10 >10 Epithelial cell‡ 0 5 5 5 5 5 5 1 - 5 6 - 10 >10 Leukocyte‡ 0 5 5 5 5 5 5 1 - 10 11 - 50 51 - 100 >100 Erythrocyte‡ 0 5 5 5 5 5 5 1 - 10 11 - 50 51 - 100 >100 Specific gravity 1.000 - 1.010 1.011 - 1.020 1.021 - 1.030 1 1.031 - 1.040 2 3 1 1.041 - 1.050 1 2 2 3 1.051 - 1.060 2 1 1 1 1 >1.060 1 4 4 ‡Sediment. mg/kg group and in some males and females in the 2000 mg/kg group. Although those symptoms were considered to be test substance related effects, these were not toxic effects since there were no changes on gastrointestinal track at necropsy and histopathology, and there were no effects on the body weights. Blood is an important index of physiological and pathological status in man and animal [25]. Some statis- tically significant differences were observed from the results of hematological parameters of female rats treated with UP446 at the dose of 2000 mg/kg/day. However, all differences in hematology parameters were not consid- ered to be test substance-related effects because of the small magnitude of the difference with in the normal ranges, the lack of a clear dose response and the lack of relationship without histological correlation. There are strong evidences to suggest that both the traditional non selective non-steroidal anti-inflammatory drugs (NSAIDs) and the selective cyclooxygenase-2 (COX-2) inhibitors are associated with an increased risk of thrombotic events and adverse gastrointestinal effects [26,27]. To confirm the impact of UP466 on blood coagulation, we measured pro-thrombin and activated partial throm- boplastin times after 26-week of repeated oral dose of rats at dose rate as high as 2000 mg/kg/day, an equivalent of 22.68 g average human daily dose. There was no Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 21 Table 4. Urinalysis results of UP446 for 26-week repeated oral dose toxicity with 4-week recovery period in female rats. Parameters Grade Main group (mg/kg) Recovery group (mg/kg) Group/dose (mg/kg) Control UP446, 500 UP446, 1000UP446, 2000 Control UP446, 2000 No. of animals 5 5 5 5 5 5 Volume (mL) 8.2 ± 2.5 6.6 ± 2.76.6 ± 3.5 9.0 ± 2.8 6.9 ± 2.6 9.1 ± 4.5 Color Pale yellow 5 Yellow 5 5 5 5 5 Transparency Clear 5 5 5 5 5 4 Mild turbidity Turbidity 1 pH 5 6 1 1 6.5 1 7 1 3 2 2 8 1 4 1 1 1 9 3 1 3 1 1 2 Protein (mg/dL) 5 2 5 5 2 1 25 3 3 2 75 2 150 500 Glucose (mg/dL) Normal 5 5 5 5 5 5 50 100 300 1000 Ketone body (mg/dL) 5 5 5 5 5 5 5 15 50 100 Bilirubin (mg/dL) 5 5 5 5 5 5 1 3 4 Occult blood (Ery/μL) 5 5 5 5 5 5 10 25 50 150 250 Cast‡ 0 5 5 5 5 5 5 1 - 5 6 - 10 >10 Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 22 Continued Epithelial cell‡ 0 5 5 5 5 5 5 1 - 5 6 - 10 >10 Leukocyte‡ 0 5 5 5 5 5 5 1 - 10 11 - 50 51 - 100 >100 Erythrocyte‡ 0 5 5 5 5 5 5 1 - 10 11 - 50 51 - 100 >100 Specific gravity 1.000 - 1.010 1.011 - 1.020 1.021 - 1.030 1 1 1 1.031 - 1.040 4 2 2 2 1 1 1.041 - 1.050 1 1 3 2 1 1.051 - 1.060 1 1 1 2 >1.060 1 1 ‡Sediment. Table 5. Mean hematological parameters of UP446 for 26-week repeated oral dose toxicity with 4-week recovery period in male rats. Main group (mg/kg) Recovery group (mg/kg) Parameters Control UP446, 500 UP446, 1000 UP446, 2000 Control UP446, 2000 RBC (×106 cells/μL) 8.74 ± 0.26 8.55 ± 0.23 8.64 ± 0.35 8.61 ± 0.38 8.57 ± 0.16 8.69 ± 0.34 HGB (g/dL) 14.8 ± 0.6 14.6 ± 0.6 14.8 ± 0.7 14.9 ± 0.5 14.6 ± 0.5 15.2 ± 0.5 HCT (%) 45.8 ± 2.0 44.7 ± 1.7 45.4 ± 2.0 45.1 ± 1.4 44.5 ± 1.3 45.6 ± 1.6 MCV (fL) 52.4 ± 1.9 52.3 ± 2.1 52.6 ± 1.5 52.4 ± 1.5 52.0 ± 2.2 52.5 ± 1.0 MCH (pg) 17.0 ± 0.5 17.1 ± 0.7 17.2 ± 0.5 17.3 ± 0.5 17.1 ± 0.8 17.6 ± 0.3 MCHC (g/dL) 32.4 ± 0.5 32.8 ± 0.5 32.7 ± 0.6 33.0 ± 0.4 32.9 ± 0.3 33.4 ± 0.2* PLT (×103 cells/μL) 1068 ± 93 1083 ± 87 1092 ± 156 1135 ± 98 1158 ± 138 1051 ± 92 Reti (%) 2.2 ± 0.3 2.3 ± 0.5 2.2 ± 0.8 2.0 ± 0.4 2.5 ± 0.3 2.3 ± 0.4 WBC (×103 cells/μL) 7.13 ± 0.81 6.96 ± 2.16 6.53 ± 1.47 6.58 ± 1.87 6.98 ± 2.47 6.67 ± 1.61 NEU 20.1 ± 3.7 20.7 ± 9.0 17.8 ± 4.9 24.6 ± 6.1 26.3 ± 6.1 14.9 ± 2.0** LYM 72.5 ± 4.5 72.2 ± 9.8 76.2 ± 5.2 69.1 ± 6.2 67.0 ± 5.8 78.1 ± 1.9** MONO 3.7 ± 1.0 3.8 ± 1.6 2.8 ± 1.2 3.0 ± 0.8 4.0 ± 0.9 4.1 ± 1.1 EOS 1.3 ± 0.4 1.3 ± 0.4 1.1 ± 0.2 1.1 ± 0.3 1.4 ± 0.6 1.6 ± 0.8 BASO 0.3 ± 0.1 0.3 ± 0.1 0.3 ± 0.1 0.3 ± 0.1 0.3 ± 0.1 0.2 ± 0.1 PT (sec) 15.9 ± 0.6 16.1 ± 0.6 15.6 ± 1.1 15.8 ± 0.7 16.3 ± 0.4 16.0 ± 1.0 APTT (sec) 16.6 ± 0.9 16.5 ± 1.2 16.4 ± 1.7 16.3 ± 1.2 17.1 ± 1.4 17.4 ± 0.9 Mean hematological parameters were calculated and data were expressed as mean ± SD (n = 10, except control group; n = 9, Recovery group; n = 5). Signifi- cantly different from control by Student t-test for recovery *p < 0.05, **p < 0.01. Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 23 Table 6. Mean hematological parameters of UP446 for 26-week repeated oral dose toxicity with 4-week recovery period in female rats. Main group (mg/kg) Recovery group (mg/kg) Parameters Control UP446, 500 UP446, 1000 UP446, 2000 Control UP446, 2000 RBC (×106 cells/μL) 7.78 ± 0.28 7.82 ± 0.30 7.71 ± 7.44 7.44 ± 0.22* 7.83 ± 0.50 7.74 ± 0.11 HGB (g/dL) 14.5 ± 0.5 14.1 ± 0.4 14.5 ± 0.4 13.7 ± 0.3** 14.8 ± 0.2 14.4 ± 0.5 HCT (%) 43.2 ± 1.8 42.3 ± 1.2 43.4 ± 1.4 41.2 ± 1.0** 43.0 ± 1.0 42.1 ± 1.6 MCV (fL) 55.6 ± 1.6 54.1 ± 1.2* 56.3 ± 1.3 55.4 ± 1.0 55.1 ± 3.0 54.4 ± 1.4 MCH (pg) 18.6 ± 0.3 18.1 ± 0.5* 18.8 ± 0.4 18.4 ± 0.3 19.0 ± 1.1 18.7 ± 0.5 MCHC (g/dL) 33.4 ± 0.5 33.4 ± 0.3 33.3 ± 0.4 33.3 ± 0.4 34.6 ± 0.9 34.3 ± 0.6 PLT (×103 cells/μL) 1029 ± 130 1149 ± 200 1049 ± 138 1011 ± 62 1053 ± 101 1022 ± 86 Reti (%) 2.0 ± 0.4 1.9 ± 0.2 2.1 ± 0.3 1.8 ± 0.4 2.2 ± 0.8 1.8 ± 0.4 WBC (×103 cells/μL) 3.13 ± 0.96 3.74 ± 1.13 3.32 ± 0.71 3.59 ± 1.04 4.82 ± 1.85 3.58 ± 1.01 NEU 19.5 ± 6.0 19.8 ± 6.8 20.5 ± 4.1 16.8 ± 3.7 16.1 ± 8.4 14.2 ± 3.3 LYM 73.6 ± 7.6 73.5 ± 7.1 72.4 ± 4.5 76.4 ± 5.3 76.3 ± 11.8 78.9 ± 2.8 MONO 3.6 ± 1.3 3.3 ± 1.1 3.6 ± 1.1 3.4 ± 1.7 3.7 ± 2.3 3.4 ± 0.8 EOS 1.5 ± 0.9 1.6 ± 0.5 1.5 ± 0.4 1.5 ± 0.5 1.4 ± 0.6 1.5 ± 0.5 BASO 0.3 ± 0.1 0.3 ± 0.1 0.3 ± 0.1 0.3 ± 0.1 0.2 ± 0.1 0.3 ± 0.2 PT (sec) 15.1 ± 1.0 15.3 ± 1.0 15.2 ± 0.7 15.5 ± 0.6 15.3 ± 0.7 15.2 ± 0.3 APTT (sec) 17.1 ± 0.8 16.8 ± 1.4 16.6 ± 0.5 17.0 ± 1.0 16.0 ± 1.7 16.4 ± 0.5 Mean hematological parameters were calculated and data were expressed as mean ± SD (n = 10, except control group; n = 9). Significantly different from con- trol by Dunnett’s t-test: *p < 0.05, **p < 0.01. Table 7. Mean clinical chemistry parameters of UP446 for 26-week repeated oral dose toxicity with 4-week recovery period in male rats. Main group (mg/kg) Recovery group (mg/kg) Parameters Control UP446, 500 UP446, 1000 UP446, 2000 Control UP446, 2000 ALT (U/L) 43.7 ± 16.5 36.6 ± 13.2 30.9 ± 5.7 35.8 ± 9.8 33.7 ± 6.9 31.5 ± 7.1 AST (U/L) 90.2 ± 24.5 88.9 ± 28.9 85.1 ± 17.5 81.7 ± 25.2 94.6 ± 26.6 110.6 ± 20.8 ALP (U/L) 190.5 ± 52.5 223.4 ± 37.4 209.7 ± 25.7 243.0 ± 43.8 225.2 ± 48.8 200.9 ± 27.0 GGT (U/L) 0.23 ± 0.14 0.40 ± 0.30 0.81 ± 0.34** 0.46 ± 0.30 1.15 ± 0.57 0.88 ± 0.39 Glu (mg/dL) 161 ± 15 154 ± 10 151 ± 10 139 ± 12** 165 ± 13 174 ± 19 BUN (mg/dL) 10.9 ± 0.8 10.4 ± 1.2 10.3 ± 1.5 9.4 ± 1.3 14.0 ± 1.5 12.1 ± 1.0* Crea (mg/dL) 0.52 ± 0.05 0.50 ± 0.04 0.47 ± 0.06 0.48 ± 0.05 0.52 ± 0.05 0.53 ± 0.04 T-Bili (mg/dL) 0.06 ± 0.03 0.06 ± 0.02 0.05 ± 0.02 0.04 ± 0.01 0.07 ± 0.02 0.07 ± 0.02 T-Chol (mg/dL) 99 ± 22 97 ± 24 88 ± 26 95 ± 35 110 ± 17 106 ± 10 TG (mg/dL) 88 ± 45 105 ± 61 106 ± 80 86 ± 44 87 ± 21 73 ± 17 TP (g/dL) 6.1 ± 0.3 6.0 ± 0.2 6.1 ± 0.3 6.1 ± 0.3 6.4 ± 0.3 6.3 ± 0.2 Alb (g/dL) 2.3 ± 0.1 2.3 ± 0.1 2.3 ± 0.1 2.5 ± 0.1 2.4 ± 0.2 2.4 ± 0.1 A/G ratio 0.61 ± 0.03 0.63 ± 0.04 0.63 ± 0.03 0.67 ± 0.07 0.62 ± 0.05 0.62 ± 0.04 P (mg/dL) 5.53 ± 0.73 5.42 ± 0.61 5.75 ± 0.62 5.47 ± 0.73 5.98 ± 0.30 5.47 ± 0.25* Ca (mg/dL) 10.3 ± 0.4 10.1 ± 0.4 10.2 ± 0.2 10.2 ± 0.2 10.1 ± 0.3 10.0 ± 0.3 Na (mmol/L) 142 ± 1 142 ± 1 143 ± 1 143 ± 1* 141 ± 1 141 ± 1 K (mmol/L) 4.7 ± 0.2 4.6 ± 0.3 4.6 ± 0.2 4.5 ± 0.2 5.0 ± 0.3 4.9 ± 0.1 Cl (mmol/L) 105 ± 1 105 ± 2 105 ± 1 105 ± 1 103 ± 1 104 ± 1 Mean clinical chemistry parameters were calculated and data were expressed as mean ± SD. Significantly different from control by Dunnett’s t-test for main group and Student t-test for recovery group: *p < 0.05, **p < 0.01. Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats Copyright © 2013 SciRes. FNS 24 Table 8. Mean clinical chemistry parameters of UP446 for 26-week repeated oral dose toxicity with 4 week recovery period in female rats. Main group (mg/kg) Recovery group (mg/kg) Parameters Control UP446, 500 UP446, 1000 UP446, 2000 Control UP446, 2000 ALT (U/L) 64.1 ± 41.3 48.4 ± 31.4 68.6 ± 83.2 60.2 ± 50.0 77.1 ± 62.3 29.7 ± 6.6 AST (U/L) 142.1 ± 89.9 105.4 ± 41.5 154.6 ± 154.3 166.5 ± 161.7 207.1 ± 162.0 98.9 ± 31.1 ALP (U/L) 92.6 ± 16.0 108.5 ± 35.9 93.6 ± 24.4 94.0 ± 16.1 81.1 ± 12.1 77.1 ± 22.3 GGT (U/L) 0.94 ± 0.34 0.78 ± 1.00 0.67 ± 0.45 0.94 ± 0.60 0.75 ± 0.43 0.37 ± 0.32 Glu (mg/dL) 162 ± 18 154 ± 14 156 ± 18 148 ± 13 170 ± 29 149 ± 7 BUN (mg/dL) 14.0 ± 1.6 13.3 ± 1.5 12.8 ± 2.7 12.8 ± 1.7 14.9 ± 1.3 13.5 ± 1.2 Crea (mg/dL) 0.55 ± 0.05 0.54 ± 0.08 0.54 ± 0.05 0.57 ± 0.08 0.57 ± 0.09 0.51 ± 0.04 T-Bili (mg/dL) 0.09 ± 0.02 0.07 ± 0.02 0.09 ± 0.02 0.08 ± 0.02 0.13 ± 0.03 0.11 ± 0.02 T-Chol (mg/dL) 116 ± 28 111 ± 19 113 ± 31 122 ± 25 107 ± 25 124 ± 19 TG (mg/dL) 32 ± 10 31 ± 9 35 ± 14 43 ± 16 52 ± 19 79 ± 25 TP (g/dL) 7.0 ± 0.4 7.0 ± 0.4 7.0 ± 0.4 7.0 ± 0.3 6.8 ± 0.4 7.1 ± 0.4 Alb (g/dL) 3.2 ± 0.2 3.2 ± 0.3 3.2 ± 0.3 3.4 ± 0.2 3.0 ± 0.1 3.2 ± 0.2 A/G ratio 0.84 ± 0.03 0.84 ± 0.09 0.84 ± 0.06 0.96 ± 0.09 0.79 ± 0.05 0.83 ± 0.04 P (mg/dL) 4.44 ± 0.82 4.35 ± 0.58 4.47 ± 0.64 4.37 ± 1.05 4.28 ± 0.36 3.82 ± 0.53 Ca (mg/dL) 10.5 ± 0.3 10.5 ± 0.4 10.6 ± 0.4 10.7 ± 0.3 10.1 ± 0.3 10.2 ± 0.2 Na (mmol/L) 142 ± 1 142 ± 1 143 ± 1 143 ± 1 141 ± 1 142 ± 1 K (mmol/L) 4.0 ± 0.3 4.0 ± 0.2 4.1 ± 0.3 4.1 ± 0.3 3.9 ± 0.2 4.0 ± 0.3 Cl (mmol/L) 105 ± 2 105 ± 3 105 ± 2 104 ± 1 104 ± 1 104 ± 1 Mean clinical chemistry parameters were calculated and data were expressed as mean ± SD. treatment related or statistically significant changes in coagulation parameters in each treatment group for both males and females, when compared to the control group. Adverse effects of traditional NSAIDs causing gastroin- testinal lesions dosed with as little ibuprofen as 2 mg/kg/ day, equivalent to approximately 140 mg/day in humans has been demonstrated in rats [23,28]. The current study substantiate this findings in that after 26-week of re- peated oral treatment of UP466 doses equivalent to as high as 22.68 g/day for average human showed no gastric mucosal or duodenal microscopic changes. These find- ings suggest that the unique blend of baicalin and cate- chin is well-tolerated by the gastrointestinal mucosa in rats and may presumably be well tolerated in humans. In this regard, UP446 is consistent with the reports of other flavonoids, for instance garcinol, rutin and quercetin, shown to either be gastro protective or have been used for the treatment of gastric ulcerations [23,29]. This study also showed that no adverse change in clinical chemistry parameters were observed in male or female rats treated with UP446. Even at the highest daily oral dosage of 2000 mg/kg, UP446 did not induce any changes of liver enzymes such as ALT, AST, ALP and caused no changes of absolute and relative liver weight and no histopathology changes of liver tissues. The sta- tistically significant changes in mean clinical chemistry for glucose in males administered 2000 mg/kg/day were not adverse or not related to expose to test substance be- cause the differences in these parameters from those of the control groups were relatively small in magnitude they are not found in both sexes. The absolute and relative organ weights in all treated groups except increased absolute adrenal gland weight at female recovery group were not significantly different from those of control group. Therefore, it was considered to be no toxicological significance since the organ did not show test substance related changes in the main group and these were secondary effects of body weight. There were no macroscopic observations considered to be treatment related in this study. No gross abnormalities 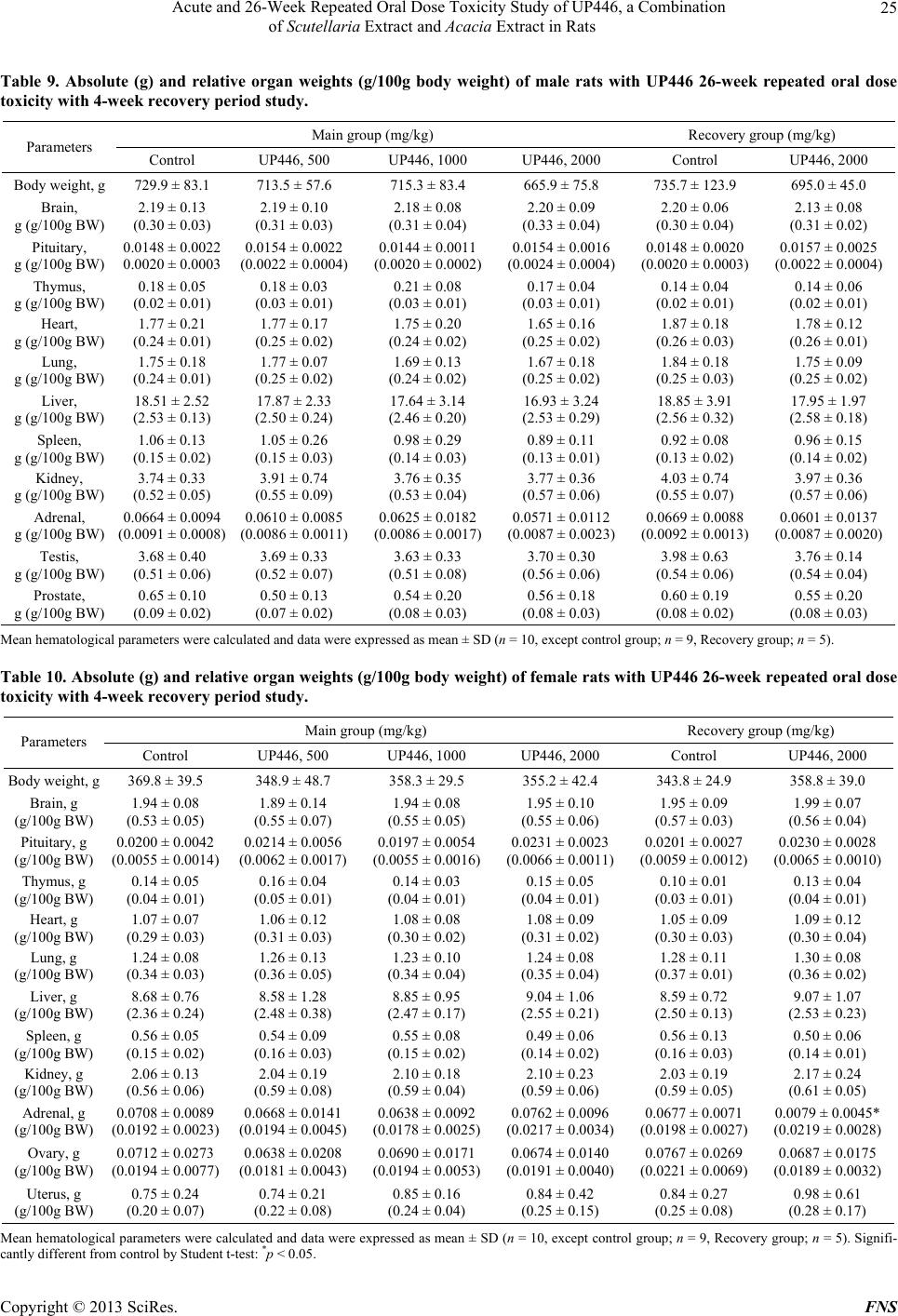 Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 25 Table 9. Absolute (g) and relative organ weights (g/100g body weight) of male rats with UP446 26-week repeated oral dose toxicity with 4-week recovery period study. Main group (mg/kg) Recovery group (mg/kg) Parameters Control UP446, 500 UP446, 1000 UP446, 2000 Control UP446, 2000 Body weight, g 729.9 ± 83.1 713.5 ± 57.6 715.3 ± 83.4 665.9 ± 75.8 735.7 ± 123.9 695.0 ± 45.0 Brain, g (g/100g BW) 2.19 ± 0.13 (0.30 ± 0.03) 2.19 ± 0.10 (0.31 ± 0.03) 2.18 ± 0.08 (0.31 ± 0.04) 2.20 ± 0.09 (0.33 ± 0.04) 2.20 ± 0.06 (0.30 ± 0.04) 2.13 ± 0.08 (0.31 ± 0.02) Pituitary, g (g/100g BW) 0.0148 ± 0.0022 0.0020 ± 0.0003 0.0154 ± 0.0022 (0.0022 ± 0.0004) 0.0144 ± 0.0011 (0.0020 ± 0.0002) 0.0154 ± 0.0016 (0.0024 ± 0.0004) 0.0148 ± 0.0020 (0.0020 ± 0.0003) 0.0157 ± 0.0025 (0.0022 ± 0.0004) Thymus, g (g/100g BW) 0.18 ± 0.05 (0.02 ± 0.01) 0.18 ± 0.03 (0.03 ± 0.01) 0.21 ± 0.08 (0.03 ± 0.01) 0.17 ± 0.04 (0.03 ± 0.01) 0.14 ± 0.04 (0.02 ± 0.01) 0.14 ± 0.06 (0.02 ± 0.01) Heart, g (g/100g BW) 1.77 ± 0.21 (0.24 ± 0.01) 1.77 ± 0.17 (0.25 ± 0.02) 1.75 ± 0.20 (0.24 ± 0.02) 1.65 ± 0.16 (0.25 ± 0.02) 1.87 ± 0.18 (0.26 ± 0.03) 1.78 ± 0.12 (0.26 ± 0.01) Lung, g (g/100g BW) 1.75 ± 0.18 (0.24 ± 0.01) 1.77 ± 0.07 (0.25 ± 0.02) 1.69 ± 0.13 (0.24 ± 0.02) 1.67 ± 0.18 (0.25 ± 0.02) 1.84 ± 0.18 (0.25 ± 0.03) 1.75 ± 0.09 (0.25 ± 0.02) Liver, g (g/100g BW) 18.51 ± 2.52 (2.53 ± 0.13) 17.87 ± 2.33 (2.50 ± 0.24) 17.64 ± 3.14 (2.46 ± 0.20) 16.93 ± 3.24 (2.53 ± 0.29) 18.85 ± 3.91 (2.56 ± 0.32) 17.95 ± 1.97 (2.58 ± 0.18) Spleen, g (g/100g BW) 1.06 ± 0.13 (0.15 ± 0.02) 1.05 ± 0.26 (0.15 ± 0.03) 0.98 ± 0.29 (0.14 ± 0.03) 0.89 ± 0.11 (0.13 ± 0.01) 0.92 ± 0.08 (0.13 ± 0.02) 0.96 ± 0.15 (0.14 ± 0.02) Kidney, g (g/100g BW) 3.74 ± 0.33 (0.52 ± 0.05) 3.91 ± 0.74 (0.55 ± 0.09) 3.76 ± 0.35 (0.53 ± 0.04) 3.77 ± 0.36 (0.57 ± 0.06) 4.03 ± 0.74 (0.55 ± 0.07) 3.97 ± 0.36 (0.57 ± 0.06) Adrenal, g (g/100g BW) 0.0664 ± 0.0094 (0.0091 ± 0.0008) 0.0610 ± 0.0085 (0.0086 ± 0.0011) 0.0625 ± 0.0182 (0.0086 ± 0.0017) 0.0571 ± 0.0112 (0.0087 ± 0.0023) 0.0669 ± 0.0088 (0.0092 ± 0.0013) 0.0601 ± 0.0137 (0.0087 ± 0.0020) Testis, g (g/100g BW) 3.68 ± 0.40 (0.51 ± 0.06) 3.69 ± 0.33 (0.52 ± 0.07) 3.63 ± 0.33 (0.51 ± 0.08) 3.70 ± 0.30 (0.56 ± 0.06) 3.98 ± 0.63 (0.54 ± 0.06) 3.76 ± 0.14 (0.54 ± 0.04) Prostate, g (g/100g BW) 0.65 ± 0.10 (0.09 ± 0.02) 0.50 ± 0.13 (0.07 ± 0.02) 0.54 ± 0.20 (0.08 ± 0.03) 0.56 ± 0.18 (0.08 ± 0.03) 0.60 ± 0.19 (0.08 ± 0.02) 0.55 ± 0.20 (0.08 ± 0.03) Mean hematological parameters were calculated and data were expressed as mean ± SD (n = 10, except control group; n = 9, Recovery group; n = 5). Table 10. Absolute (g) and relative organ weights (g/100g body weight) of female rats with UP446 26-week repeated oral dose toxicity with 4-week recovery period study. Main group (mg/kg) Recovery group (mg/kg) Parameters Control UP446, 500 UP446, 1000 UP446, 2000 Control UP446, 2000 Body weight, g 369.8 ± 39.5 348.9 ± 48.7 358.3 ± 29.5 355.2 ± 42.4 343.8 ± 24.9 358.8 ± 39.0 Brain, g (g/100g BW) 1.94 ± 0.08 (0.53 ± 0.05) 1.89 ± 0.14 (0.55 ± 0.07) 1.94 ± 0.08 (0.55 ± 0.05) 1.95 ± 0.10 (0.55 ± 0.06) 1.95 ± 0.09 (0.57 ± 0.03) 1.99 ± 0.07 (0.56 ± 0.04) Pituitary, g (g/100g BW) 0.0200 ± 0.0042 (0.0055 ± 0.0014) 0.0214 ± 0.0056 (0.0062 ± 0.0017) 0.0197 ± 0.0054 (0.0055 ± 0.0016) 0.0231 ± 0.0023 (0.0066 ± 0.0011) 0.0201 ± 0.0027 (0.0059 ± 0.0012) 0.0230 ± 0.0028 (0.0065 ± 0.0010) Thymus, g (g/100g BW) 0.14 ± 0.05 (0.04 ± 0.01) 0.16 ± 0.04 (0.05 ± 0.01) 0.14 ± 0.03 (0.04 ± 0.01) 0.15 ± 0.05 (0.04 ± 0.01) 0.10 ± 0.01 (0.03 ± 0.01) 0.13 ± 0.04 (0.04 ± 0.01) Heart, g (g/100g BW) 1.07 ± 0.07 (0.29 ± 0.03) 1.06 ± 0.12 (0.31 ± 0.03) 1.08 ± 0.08 (0.30 ± 0.02) 1.08 ± 0.09 (0.31 ± 0.02) 1.05 ± 0.09 (0.30 ± 0.03) 1.09 ± 0.12 (0.30 ± 0.04) Lung, g (g/100g BW) 1.24 ± 0.08 (0.34 ± 0.03) 1.26 ± 0.13 (0.36 ± 0.05) 1.23 ± 0.10 (0.34 ± 0.04) 1.24 ± 0.08 (0.35 ± 0.04) 1.28 ± 0.11 (0.37 ± 0.01) 1.30 ± 0.08 (0.36 ± 0.02) Liver, g (g/100g BW) 8.68 ± 0.76 (2.36 ± 0.24) 8.58 ± 1.28 (2.48 ± 0.38) 8.85 ± 0.95 (2.47 ± 0.17) 9.04 ± 1.06 (2.55 ± 0.21) 8.59 ± 0.72 (2.50 ± 0.13) 9.07 ± 1.07 (2.53 ± 0.23) Spleen, g (g/100g BW) 0.56 ± 0.05 (0.15 ± 0.02) 0.54 ± 0.09 (0.16 ± 0.03) 0.55 ± 0.08 (0.15 ± 0.02) 0.49 ± 0.06 (0.14 ± 0.02) 0.56 ± 0.13 (0.16 ± 0.03) 0.50 ± 0.06 (0.14 ± 0.01) Kidney, g (g/100g BW) 2.06 ± 0.13 (0.56 ± 0.06) 2.04 ± 0.19 (0.59 ± 0.08) 2.10 ± 0.18 (0.59 ± 0.04) 2.10 ± 0.23 (0.59 ± 0.06) 2.03 ± 0.19 (0.59 ± 0.05) 2.17 ± 0.24 (0.61 ± 0.05) Adrenal, g (g/100g BW) 0.0708 ± 0.0089 (0.0192 ± 0.0023) 0.0668 ± 0.0141 (0.0194 ± 0.0045) 0.0638 ± 0.0092 (0.0178 ± 0.0025) 0.0762 ± 0.0096 (0.0217 ± 0.0034) 0.0677 ± 0.0071 (0.0198 ± 0.0027) 0.0079 ± 0.0045* (0.0219 ± 0.0028) Ovary, g (g/100g BW) 0.0712 ± 0.0273 (0.0194 ± 0.0077) 0.0638 ± 0.0208 (0.0181 ± 0.0043) 0.0690 ± 0.0171 (0.0194 ± 0.0053) 0.0674 ± 0.0140 (0.0191 ± 0.0040) 0.0767 ± 0.0269 (0.0221 ± 0.0069) 0.0687 ± 0.0175 (0.0189 ± 0.0032) Uterus, g (g/100g BW) 0.75 ± 0.24 (0.20 ± 0.07) 0.74 ± 0.21 (0.22 ± 0.08) 0.85 ± 0.16 (0.24 ± 0.04) 0.84 ± 0.42 (0.25 ± 0.15) 0.84 ± 0.27 (0.25 ± 0.08) 0.98 ± 0.61 (0.28 ± 0.17) Mean hematological parameters were calculated and data were expressed as mean ± SD (n = 10, except control group; n = 9, Recovery group; n = 5). Signifi- antly different from control by Student t-test: *p < 0.05. c Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 26 attributed to the test article were noted for any of the euthanized animals necropsied at the end of the 26-week observation period. Incidental findings correspond to historical control values for other experiments [30,31]. In the present study, the oral lethal dose of UP446 for male and female rats is in excess of 5000 mg/kg and the no observed adverse effect level (NOAEL) of the UP446 for both male and female rats is considered to be greater than 2000 mg/kg/day. Based on our present study, further toxicity study using beagle dogs with UP446 should be also conducted in GLP institute. 5. Acknowledgements This study was funded by Chungcheong Leading Indus- try Promotion Project of the Korean Ministry of Know- eldge Economy. The authors would like to express their best gratitude to Dr. Edward Cannon, Dr. Doug Bradley, Dr. Wenwen Ma, Dr. Padma Abeysinghe, Dr. Min Chu and Unigen team for their incalculable support for the completion of this research. REFERENCES [1] M. Modak, P. Dixit, J. Londhe, S. Ghaskadbi and T. P. Devasagayam, “Indian Herbs and Herbal Drugs Used for Treatment of Diabetes,” Journal of Clinical Biochemistry and Nutrition, Vol. 40, No. 3, 2007, pp. 163-173. doi:10.3164/jcbn.40.163 [2] R. Morphy, C. Kay and Z. Rankovic, “From Magic Bul- lets to Designed Multiple Ligands,” Drug Discovery To- day, Vol. 9, No. 15, 2004, pp. 641-651. doi:10.1016/S1359-6446(04)03163-0 [3] P. A. Tatke, I. S. Nidhiya and S. G. Deshpande, “Safety Profile of Polyherbal Formulation in Female Rats by Sub- chronic Oral Ttoxicity Study,” Toxicology International, Vol. 19, No. 2, 2012, pp. 106-111. doi:10.4103/0971-6580.97196 [4] P. G. Shekelle, S. C. Morton, M. J. Suttorp, N. Buscemi and C. Friesen, “Challenges in Systematic Reviews of Complementary and Alternative Medicine Topics,” An- nals of Internal Medicine, Vol. 142, No. 12, 2005, pp. 1042-1047. doi:10.7326/0003-4819-142-1 2_Par t _2 - 200506211-00003 [5] R. Kroes and R. Walker, “Safety Issues of Botanicals and Botanical Preparations in Functional Foods,” Toxicology, Vol. 198, No. 1-3, 2004, pp. 213-220. doi:10.1016/j.tox.2004.01.028 [6] E. Middleton, C. Kandaswami and T. C. Theoharides, “The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Can- cer,” Pharmacological Review, Vol. 52, No. 4, 2000, pp. 673-751. [7] M. M. Cowan, “Plant Products as Antimicrobial Agents,” Clinical Microbiology Reviews, Vol. 12, No. 4, 1999, pp. 564-582. [8] P. Knekt, J. Kumpulainen, R. Jarvinen, H. Rissanen, M. Heliovaara, A. Reunanen, T. Hakulinen and A. Aromaa, “Flavonoid Intake and Risk of Chronic Diseases,” The American Journal of Clinical Nutrition, Vol. 76, No. 3, 2002, pp. 560-568. [9] L. Fu, B. T. Gan, Y. Zhang, X. R. Xu, E. Q. Xia and H. B. Li, “Total Phenolic Contents and Antioxidant Capacities of Herbal and Tea Infusions,” International Journal of Molecular Sciences, Vol. 12, No. 4, 2011, pp. 2112-2124. doi:10.3390/ijms12042112 [10] Y. J. Moon, X. Wang and M. E. Morris, “Dietary Flavon- oids: Effects on Xenobiotic and Carcinogen Metabolism,” Toxicological in Vitro, Vol. 20, No. 2, 2006, pp. 187-210. doi:10.1016/j.tiv.2005.06.048 [11] T. G. Razina, S. N. Udintsev, I. I. Titutrin, T. G. Bo- rovskaia and K. V. Iaremenko, “The Role of Thrombo- cyte Aggregation Function in the Mechanism of the An- timetastatic Action of an Extract of Baikal Skullcap,” Vo- prosy Onkologii, Vol. 35, No. 3, 1989, pp. 331-335. [12] N. Mahmood, C. Pizza, R. Aquino, N. De Tommasi, S. Piacente, S. Colman, A. Burke and A. J. Hay, “Inhibition of HIV Infection by Flavonoids,” Antiviral Research, Vol. 22, No. 2-3, 1993, pp. 189-199. doi:10.1016/0166-3542(93)90095-Z [13] I. M. Van Loon, “The golden Root: Rlinical Applications of Scutellaria Baicalensis GEORGI Flavonoids as Modu- lators of the Inflammatory Response,” Alternative Medi- cine Review, Vol. 2, No. 6, 1992, pp. 472-480. [14] B. H. Lee, S. J. Lee, T. H. Kang, D. H. Kim, D. H. Sohn, G. I. Ko and Y. C. Kim, “Baicalein: An in Vitro Antige- notoxic Compound from Scutellaria Baicalensis,” Planta Medica, Vol. 66, No. 1, 2000, pp. 70-71. doi:10.1055/s-0029-1243111 [15] S. Shigeta, “Recent Progress in Antiviral Chemotherapy for Respiratory Syncytial Virus Infections,” Expert Opi- nion on Investigational Drugs, Vol. 9, No. 2, 2000, pp. 221-235. doi:10.1517/13543784.9.2.221 [16] E. De Clercq, “Current Lead Natural Products for the Chemotherapy of Human Immunodeficiency Virus (HIV) Infection,” Medicinal Research Reviews, Vol. 20, No. 5, 2000, pp. 323-349. doi:10.1002/1098-1128(200009)20:5<323::AID-MED1>3 .0.CO;2-A [17] Y. Huang, S. Y. Tsang, X. Yao and Z. Y. Chen, “Bio- logical Properties of Baicalein in Cardiovascular Sys- tem,” Current Drug Targets Cardiovascular & Haema- tological Disorder, Vol. 5, No. 2, 2005, pp. 177-184. doi:10.2174/1568006043586206 [18] B. P. Burnett, Q. Jia, Y. Zhao and R. M. Levy, “ A Me- dicinal Extract of Scutellaria baicalensis and Acacia cat- echu Acts as a Dual Inhibitor of Cyclooxygenase and 5- Lipoxygenase to Reduce Inflammation,” Journal of Me- dicinal Food, Vol. 10, No. 3, 2007, pp. 442-451. doi:10.1089/jmf.2006.255 [19] S. L. Morgan, J. E. Baggott, L. Moreland, R. Desmond and A. C. Kendrach, “The Safety of Flavocoxid, a Medi- cal Food, in the Dietary Management of Knee Osteoar- thritis,” Journal of Medicinal Food, Vol. 12, No. 5, 2009, pp. 1143-1148. doi:10.1089/jmf.2008.0244 Copyright © 2013 SciRes. FNS  Acute and 26-Week Repeated Oral Dose Toxicity Study of UP446, a Combination of Scutellaria Extract and Acacia Extract in Rats 27 [20] J. S. Sampalis and L. A. Brownell, “A Randomized, Dou- ble Blind, Placebo and Active Comparator Controlled Pi- lot Study of UP446, a Novel Dual Pathway Inhibitor Anti-Inflamatory Agent of Botanical Origin,” Nutrition Journal, Vol. 11, 2012, p. 21. [21] B. P. Burnett, S. Silva, M. H. Mesches, S. Wilson and Q. Jia, “Safety Evaluation of a Combination, Defined Extract of Scutellaria Baicalensis and Acacia Catechu,” Journal of Food Biochemistry, Vol. 31, No. 6, 2007, pp. 797-825. doi:10.1111/j.1745-4514.2007.00142.x [22] M. Yimam, L. Brownell, M. Hodges and Q. Jia, “Analge- sic Effects of Standardized Bioflavonoid Composition from Scutellaria Baicalensis and Acacia Catechu,” Jour- nal of Dietary Supplements, Vol. 9, No. 3, 2012, pp. 155- 165. doi:10.3109/19390211.2012.708713 [23] M. Yimam, Y. Zhao, W. Ma, Q. Jia, S. G. Do and J. H. Shin, “90 Day Oral Toxicity Study of UP446, a Combi- nation of Defined Extracts of Scutellaria Baicalensis and Acacia Catechu, in Rats,” Food and Chemical Toxicology, Vol. 48, No. 5, 2012, pp. 1202-1209. doi:10.1016/j.fct.2010.02.011 [24] Q. Jia, “Formulation of a Mixture of Free-B-Ring Fla- vonoids and Flavans as a Therapeutic Agent,” US Patent No. 7514469, 2009. [25] A. A. Adeneyea, O. P. Ajagbonna, T. I. Adeleke and S. O. Bello, “Preliminary Toxicity and Phytochemical Studies of the Stem Bark Aqueous Extract of Musanga Cecro- pioides in Rats,” Journal of Ethnopharmacology, Vol. 105, No. 3, 2006, pp. 374-379. doi:10.1016/j.jep.2005.11.027 [26] C. Bombardier, L. Laine, A. Reicin, D. Shapiro, R. Bur- gos-Vargas, B. Davis, R. Day, M. B. Ferraz, C. J. Haw- key, M. C. Hochberg, T. K. Kvien and T. J. Schnitzer, “Comparison of upper Gastrointestinal Toxicity of Rofe- coxib and Naproxen in Patients with Rheumatoid Arthri- tis,” The New England Journal of Medicine, Vol. 343, No. 21, 2000, pp. 1520-1528. doi:10.1056/NEJM200011233432103 [27] R. S. Bresalier, V. E. Friedewald Jr., R. E. Rakel, W. C. Roberts and G. W. Williams, “The Editor’s Roundtable: Cyclooxygenase-2 Inhibitors and Cardiovascular Risk,” The American Journal of Cardiology, Vol. 96, No. 11, 2005, pp. 1589-1604. doi:10.1016/j.amjcard.2005.09.069 [28] G. A. Elliott, A. Purmalis, D. A. VanderMeer and R. H. Denlinger, “The Propionic Acids. Gastrointestinal Toxic- ity in Various Species,” Toxicologic Pathology, Vol. 16, No. 2, 1988, pp. 245-250. doi:10.1177/019262338801600217 [29] K. S. Mota, G. E. Dias, M. E. Pinto, A. Luiz-Ferreira, A. R. Souza-Brito, C. A. Hiruma-Lima, J. M. Barbosa-Filho and L. M. Batista, “Flavonoids with Gastroprotective Ac- tivity,” Molecules, Vol. 14, No. 3, 2009, pp. 979-1012. doi:10.3390/molecules14030979 [30] A. A. Adeneyea, O. P. Ajagbonna, T. I. Adeleke and S. O. Bello, “Preliminary Ttoxicity and Phytochemical Studies of the Stem Bark Aqueous Extract of Musanga Cecro- pioides in Rats,” Journal of Ethnopharmacology, Vol. 105, No. 3, 2006, pp. 374-379. doi:10.1016/j.jep.2005.11.027 [31] L. Johnson, C. S. Petty and W. B. Neaves, “A Compara- tive Study of Daily Sperm Production and Testicular Com- position in Humans and Rats,” Biology of Reproduction, Vol. 22, No. 5, 1980, pp. 1233-1243. Copyright © 2013 SciRes. FNS
|