Paper Menu >>
Journal Menu >>
 Modeling and Numerical Simulation of Material Science, 2013, 3, 16-19 Published Online January 2013 (http://www.SciRP.org/journal/mnsms) Copyright © 2013 SciRes. MNSMS Electronic and Optical Properties of Rocksalt CdO: A first-Principles Density-Functional Theory Study Gang Yao1,2, Xinyou An1, Hongwen Lei1, Yajun Fu1, Weidong Wu1,2 Science and Technology on Plasma physics Laboratory, Research Center of Laser Fusion, CAEP, P.O.Box 919-983, Mianyang 621900, P.R. China State Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials, School of Materials Science and Engineering, Southwest University of Science and Technology, Mianyang 621010, P.R. China Email: wuweidongding@163.com Received 2012 ABSTRACT The structural, electronic and optical properties of rocksalt CdO have been studied using the plane-wave -based pseudo- potential density functional theory within generalized gradient approximation. The calculated lattice parameters are in agreement with previous experimental work. The band structure, density of states, and Mulliken charge population are obtained, which indicates that rocksalt CdO having the properties of a halfmetal due to an indirect band gap of -0.51eV. The mechanical properties show that rocksalt CdO is mechanically stable, isotropic and malleable. Significantly, we propose a correct value for ε1(0) of about 4.75, which offers theoretical data for the design and application for rocksalt CdO in optoelectronic materials. Keywords: Densit y-Functional Theory; Electronic Structure; Optical Properties; Rocksalt CdO 1. Introduction Recently, electronic structure and optical properties of transparent conductive oxides (TCOS) such as cadmium oxide (CdO) are of tremendously increasing interest, in response to the industrial demand for semiconductor photoelectric device operating in solar cell, liquid-cr ystal displays (LCD), gas sensor, electrochromic devices and ultraviolet semiconductor laser [ 1, 2]. CdO has various polymorphs such as Pm3m ( 1 h O ), F-43m ( 2 d T ) and P63mc ( 4 6v C ), and it crystallizes in the rocksalt structure wit h Fm -3m ( 5 h O ) space group at room temperature. However, on applying pressure up to 89GPa [3] (90. 6 GPa [4]), rocksalt CdO undergoes a structural phase transition to CsCl-type with Pm3m ( 1 h O ). Several experimental as well as quite a few ab initio theoretical studies involving method of local density ap- proximation (LDA) [5], Hartree-Fock method [6], full potential linearized augmented plane wave (FP-LAPW ) [3] and other calculations based on density functional theory (DFT) have been performed to study the elastic and electronic properties of the rocksalt CdO over the past two decades [2,3,5-9]. Schleife et al. have reported the complex dielectric function and predicted a static dielectric function ε1(0) of 7.20 [5]. As we all known, howe ver, the DFT-GGA scheme tends to underestimate slightly the bonding in the considered group-II oxide polymorphs [3,5], i.e., the prediction for ε1(0) is not reliable. In order to understand the relevant phenomena and design process of new materials, it is necessary to insight into the electronic and optical properties of rocksalt CdO by the theoretical analysis. So we have made a correction to optical with scissors operator [1 0] on the basic of the calculated band gap. And many useful results have been obtained, which offers theoretical data for the design and application for rocksalt CdO in optoelectronic materials. 2. Computational Details The ab initio calculations were performed by employing plane -wave ultrasoft pseudopotential and implemented in the most recent version of CASTEP code [11]. The ex- change and correlation function were given by genera- lized gradient approximation (GGA) with Per- dew-Burke -Ernze r hof ,a nd Perdew and Wang (PW91) [12]. The valence-electron configurations for the ele- ments of rocksalt CdO are Cd 4d105s2 and O 2s22p4. The cutoff energy of 600eV was employed through-out the calculation which was tested to be fully converged with respect to total energy for different volumes. The Bril- louin-zone sampling mesh parameters for the k-point set were 6×6×6 [13]. In addition, this set of parameters as- sures were implemented: the total energy tolerance of 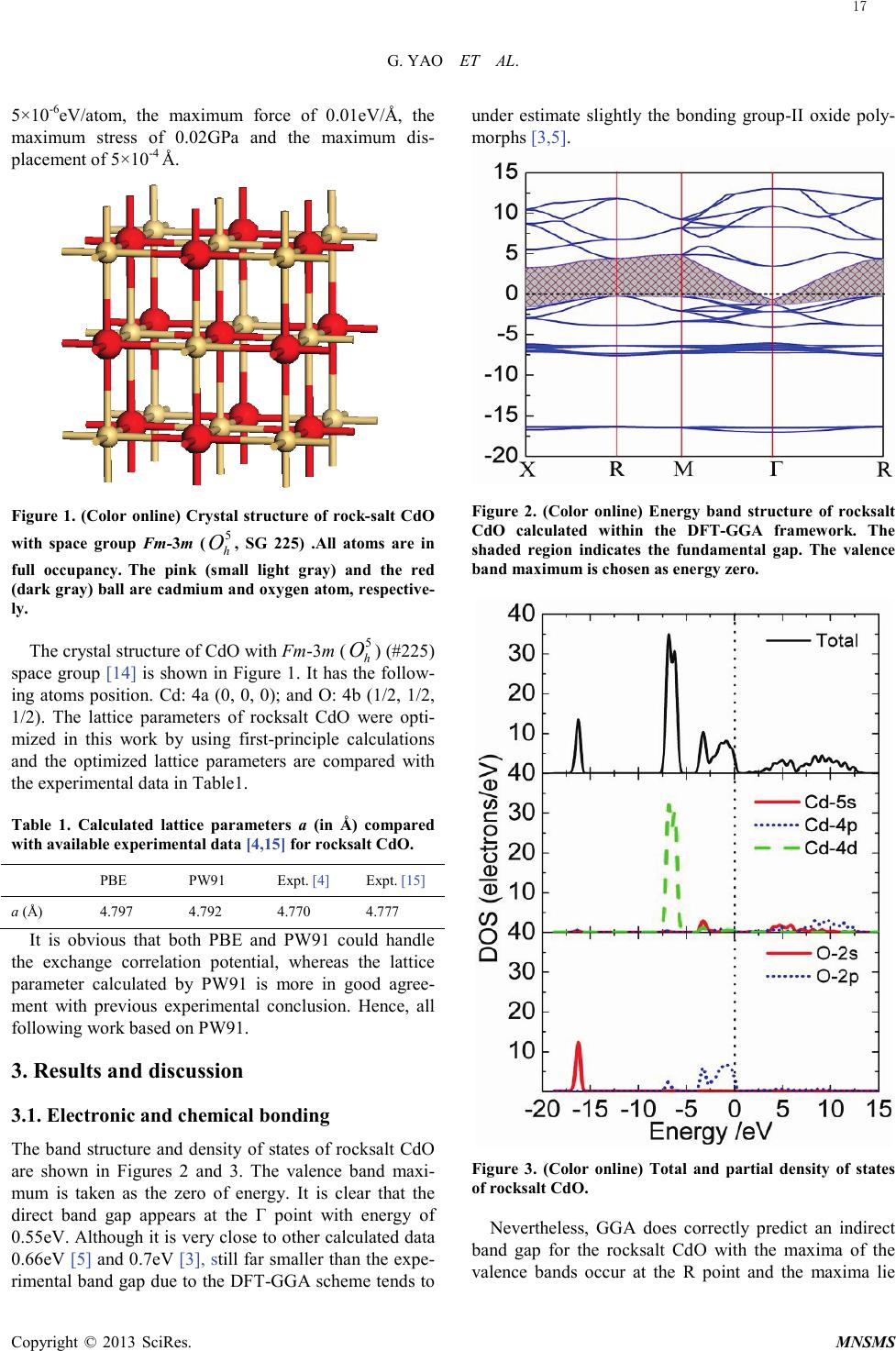 G. YAO ET AL. Copyright © 2013 SciRes. MNSMS 5×10-6eV/atom, the maximum force of 0.01eV/Å, the maximum stress of 0.02GPa and the maximum dis- placement of 5×10-4 Å. Figure 1. (Color online) Crystal structure of rock-salt CdO with space group Fm -3m ( 5 h O , SG 225) .All atoms are in full occupancy. The pink (small light gray) and the red (dark gray) ball are cadmium and oxygen atom, respective- ly. The crystal structure of CdO with Fm-3m ( 5 h O ) (#225) space group [14] is shown in Figure 1. It has the follow- ing atoms position. Cd: 4a (0, 0, 0); and O: 4b (1/2, 1/2, 1/2). The lattice parameters of rocksalt CdO were opti- mized in this work by using first-principle calculations and the optimized lattice parameters are compared with the experimental data in Table1. Table 1. Calculated lattice parameters a (in Å) compared with available experimental data [4,15] for rocksalt CdO. PBE PW9 1 Expt. [4] Expt. [15] a (Å) 4.797 4.792 4.770 4.777 It is obvious that both PBE and PW91 could handle the exchange correlation potential, whereas the lattice parameter calculated by PW91 is more in good agree- ment with previous experimental conclusion. Hence, all following work based on PW91. 3. Results and discussion 3.1. Electronic and chemical bonding The band structure and density of states of rocksalt CdO are shown in Fig ures 2 and 3. The valence band maxi- mum is taken as the zero of energy. It is clear that the direct band gap appears at the Γ point with energy of 0.55eV. Although it is very close to other calculated data 0.66eV [5] and 0.7eV [3], still far smaller than the expe- rimental band gap due to the DFT-GGA scheme tends to under estimate slightly the bonding group-II oxide poly- morphs [3,5]. Figure 2. (Color online) Energy band structure of rocksalt CdO calculated within the DFT-GGA framework. The shaded region indicates the fundamental gap. The valence band maximum is chosen as energy zero. Figure 3. (Color online) Total and partial density of states of rocksalt CdO. Nevertheless, GGA does correctly predict an indirect band gap for the rocksalt CdO with the maxima of the valence bands occur at the R point and the maxima lie 17 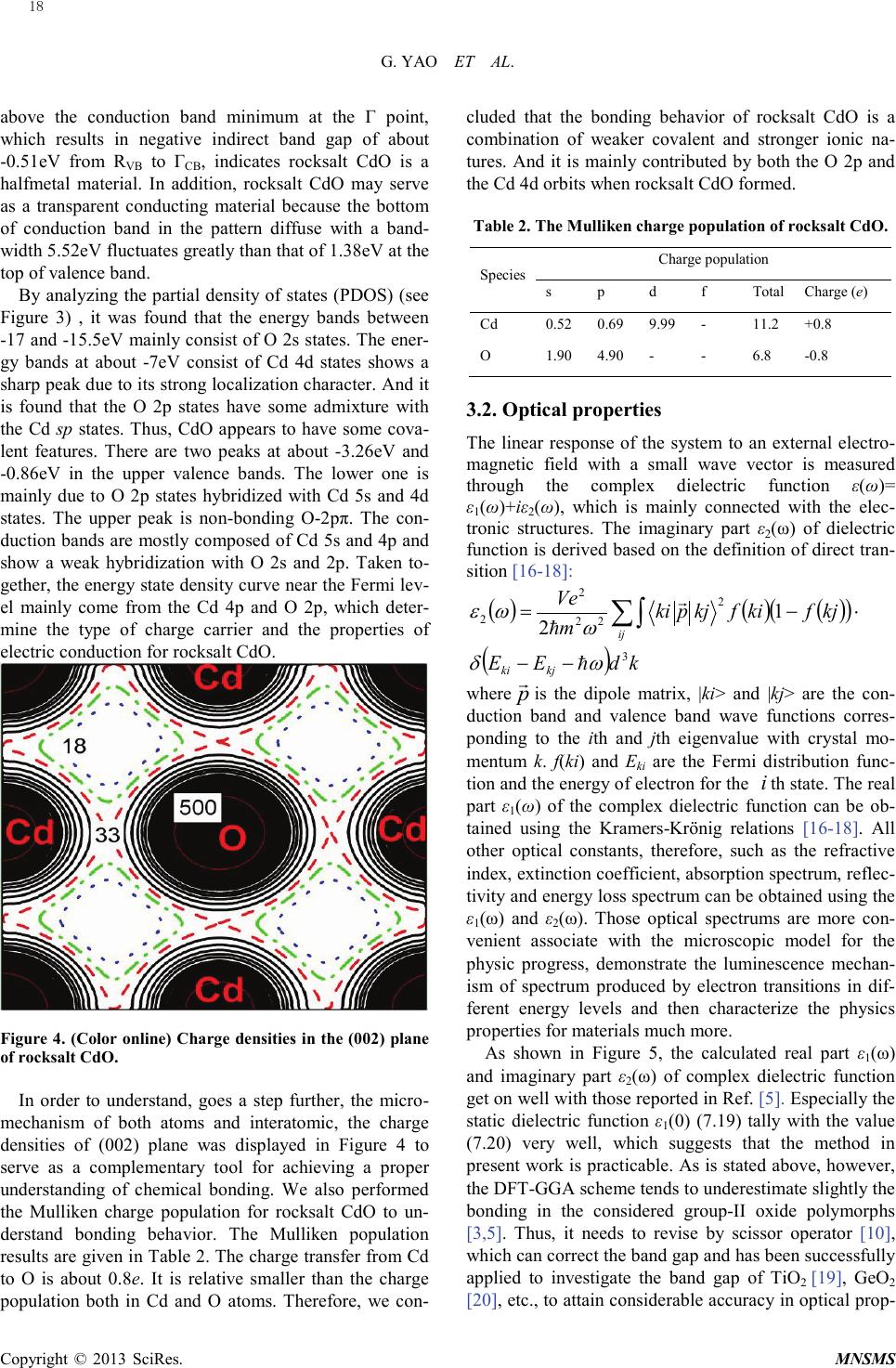 G. YAO ET AL. Copyright © 2013 SciRes. MNSMS above the conduction band minimum at the Γ point, which results in negative indirect band gap of about -0.51eV from RVB to ΓCB, indicates rocksalt CdO is a halfmetal material. In addition, rocksalt CdO may serve as a transparent conducting material because the bottom of conduction band in the pattern diffuse with a band- width 5.52eV fluctuates greatly than that of 1.38eV at the top of valence band. By analyzing the partial density of states (PDOS) (see Figure 3) , it was found that the energy bands between -17 and -15.5eV mainly consist of O 2s states. The ener- gy bands at about -7eV consist of Cd 4d states shows a sharp peak due to its strong localization character. And it is found that the O 2p states have some admixture with the Cd sp states. Thus, CdO appears to have some cova- lent features. There are two peaks at about -3.26eV and -0.86eV in the upper valence bands. The lower one is mainly due to O 2p states hybridized with Cd 5s and 4d states. The upper peak is non-bonding O-2pπ. The con- duction bands are mostly composed of Cd 5s and 4p and show a weak hybridization with O 2s and 2p. Taken to- gether, the energy state density curve near the Fermi lev- el mainly come from the Cd 4p and O 2p, which deter- mine the type of charge carrier and the properties of electric conduction for rocksalt CdO. Figure 4. (Color online) Charge densities in the (002) plane of rocksalt CdO. In order to understand, goes a step further, the micro- mechanism of both atoms and interatomic, the charge densities of (002) plane was displayed in Figure 4 to serve as a complementary tool for achieving a proper understanding of chemical bonding. We also performed the Mulliken charge population for rocksalt CdO to un- derstand bonding behavior. The Mulliken population results are given in Table 2. The charge transfer from Cd to O is about 0.8e. It is relative smaller than the charge population both in Cd and O atoms. Therefore, we con- cluded that the bonding behavior of rocksalt CdO is a combination of weaker covalent and stronger ionic na- tures. And it is mainly contributed by both the O 2p and the Cd 4d orbits when rocksalt CdO formed. Table 2. The Mulliken charge population of rocksalt CdO. Spec i es Charge population s p d f Tota l Charge (e) Cd 0.52 0.69 9.99 - 11.2 +0 .8 O 1.90 4.90 - - 6.8 -0.8 3.2. Optical properties The linear response of the system to an external electro- magnetic field with a small wave vector is measured through the complex dielectric function ε(ω)= ε1(ω)+iε2(ω), which is mainly connected with the elec- tronic structures. The imaginary part ε2(ω) of dielectric function is derived based on the definition of direct tran- sition [16-18]: ( )( )( )() ( ) kdEE kjfkifkjpki m Ve kjki ij 3 2 22 2 2 1 2 ωδ ω ωε −− ⋅−= ∑∫ whe r e p is the dipole matrix, |ki> and |kj> are the con- duction band and valence band wave functions corres- ponding to the ith and jth eigenvalue with crystal mo- mentum k. f(ki) and Eki are the Fermi distribution func- tion and the energy of electron for the i th state. The real part ε1(ω) of the complex dielectric function can be ob- tained using the Kramers-Krönig relations [16-18]. All other optical constants, therefore, such as the refractive index, extinction coefficient, absorption spectrum, refl ec- tivity and energy loss spectrum can be obtained using the ε1(ω) and ε2(ω). Those optical spectrums are more con- venient associate with the microscopic model for the physic progress, demonstrate the luminescence mechan- ism of spectrum produced by electron transitions in dif- ferent energy levels and then characterize the physics properties for materials much more. As shown in Fi gure 5, the calculated real part ε1(ω) and imaginary part ε2(ω) of complex dielectric function get on well with those reported in Ref. [5]. Especially the static dielectric function ε1(0) (7.19) tally with the value (7.20) very well, which suggests that the method in present work is practicable. As is stated above, however, the DFT-GGA scheme tends to underestimate slightly the bonding in the considered group-II oxide polymorphs [3,5]. Thus, it needs to revise by scissor operator [10] , which can correct the band gap and has been successfully applied to investigate the band gap of TiO2 [19 ], GeO2 [20], etc., to attain considerable accuracy in optical prop- 18  G. YAO ET AL. Copyright © 2013 SciRes. MNSMS erties. The modified results by scissor operator (set to 1.75(∆=Egexp-Egcal=2.3-0.55)) were plot with dotted lines in Figur e 5. The absorption edge is about 2.23eV corres- pondence with experimental band gap 2.3eV [21], i.e., it is necessary and reasonable to correct optical properties by scissor operator. In this way, we proposes a prediction value ε1(0)=4.75. Unfortunately, to our knowledge, there is few experimental and theoretical data of ε1(0) for our comparison. The real parts ε1(ω) increased rapidly with the increasing frequency where the photon energy is less than 2.82eV. The calculated maximum of the ε1(ω) is about 7.04. The imaginary part ε2(ω) becomes steeper with an increasing photon energy, which could obtain a conclusion that the rocksalt CdO could be used as poten- tial TCOS materials. Figure 5. (Color online) Real part ε 1(ω) and imaginary part ε 2(ω) of the dielectric function ε (ω) of rocksalt CdO. 4. Conclusion In summary, we calculated the structural parameters, electronic structure, and optical properties for rocksalt CdO by means of the DFT within the GGA. Our struc- tural parameters are in agreement with the previous ex- perimental date. The electronic structures revealed that the top of the valence band and the bottom of the con- duction band are decided by O 2p and Cd 4p states, re- spectively, and that CdO is a halfmetal material pre- sented a negative indirect band gap at 2.08 eV in the RVB-ΓCB direction. Significantly, a more accurate predic- tion for ε1(0) of about 4.75 was obtained by using the scissor operator on the basic of our calculated band gap, suggesting that rocksalt CdO could be used as a potential TCOS materials. However, there is no experimental data available related to the rocksalt CdO. Hence, careful ex- perimental investigations are required in order to clarify the electronic band structure and optical properties of rocksalt CdO in detail. REFERENCES [1] J.S. Tse, D.D. Klug, S. Desgreniers, J.S. Smith, R. Dutri- sac, EPL (Europhysics Letters) 86 (2009) 56001. [2] P.D.C. King, T.D. Veal, A. Schleife, J. Zúñiga-Pérez, B. Martel, P.H. Jefferson, F. Fuchs, V. Muñoz-Sanjosé, F. Bechstedt, C. F. McConville, Phys. Rev. B 79 (2009) 205205. [3] R.J. Guerrero-Moreno, N. Takeuchi, Phys. Rev. B 66 (2002) 205205. [4] H. Liu, H. Mao, M. Somayazulu, Y. Ding, Y. Meng, D. Häusermann, Phys. Rev. B 70 (2004) 094114. [5] A. Schleife, F. Fuchs, J. Furthmüller, F. Bechstedt, Phys. Rev. B 73 (2008) 245212. [6] J.E. Jaffe, R. Pandey, A.B. Kunz, Phys. Rev. B 43 (1991) 14030. [7] J. Zhang, Phys. Chem. Miner. 26 (1999) 644-648. [8] M. Durandurdu, EPL (Europhysics Letters) 84 (2008) 66003. [9] L.F.J. Piper, A. DeMasi, K.E. Smith, A. Schleife, F. Fuchs, F. Bechstedt, J. Zuniga-Perez, V. Munoz-Sanjosé, Phys. Rev. B 77 (2008) 125204. [10] P. Dufek, P. Blaha, K. Schwarz, Phys. Rev. B 50 (1994) 7279. [11] M.D. Segall, P.J.D. Lindan, M.J. Probert, C.J. Pickard, P.J. Hasnip, S.J. Clark, M.C. Payne, J. Phys.: Condens. Matter 14 (2002) 2717. [12] Y. Wang, J.P. Perdew, Phys. Rev. B 44 (1991) 13298. [13] H.J. Monkhorst, J.D. Pack, Phys. Rev. B 13 (1976) 5188. [14] Cimino, M. Marezio, J. Phys. Chem. Solids 17 (1960) 57-64. [15] F. Birch, Phys. Rev. 71 ( 1947) 809. [16] X.C. Shen, The Spectrum and Optical Property of Semi- conductor, Science Press, Beijing, 1992. [17] C.M.I. Okoye, J. Phys.: Condens. Matter 15 (2003) 5945. [18] M.Q. Cai, Z. Yin, M.S. Zhang, Appl. Phys. Lett. 83 (2003) 2805. [19] R. Asahi, Y. Taga, W. Mannstadt, A. J. Freeman, Phys. Rev. B 61 (2000) 7459. [20] Q.J. Liu, Z.T. Liu, L.P. Feng, H. Tian, Solid State Sci. 12 (2010) 1748. [21] F.P. Koffyberg, Phys. Rev. B 13 (1976) 447 19 |

