Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 10, No.2, pp.111-126, 2011 jmmce.org Printed in the USA. All rights reserved 111 Acidulation and Regeneration of Bamboo Deri ved Sorbents for Gas Phase Adsorption of Elemental Mercury Naved Siddiqui and Jarlen Don* Department of Mechanical Engineering and Energy Processes Southern Illinois University, Carbodnale, IL 62901-6603 *Corresponding Author – Email: jdon@siu.edu. Tel: 618-453-7004 ABSTRACT This paper presents results that illustrate the recycling of a bamboo derived sorbent used for the capture of elemental mercury (Hg0). The bamboo derived sorbent used is essentially a HCl functionalized activated carbon prepared from carbonization and CO2 activation of raw bamboo, that could potentially provide an alternative way to existing methods in removing mercury from flue gases from coal-fired plants. In this study, the bamboo derived sorbents were tested in a batch test using a mercury permeation tube as the source and nitrogen as a carrier gas. The recycling or regeneration of an activated carbon is an important issue to address from a coal-fired power plant point of view, and an attempt has been made to test the behavior of bamboo derived sorbents with various treatments including carbonized, carbonized-activated, carbonized-activated-acidulated, and then a follow-up recycled run after sample treatments in gas phase. From the study, it was found that bamboo derived activated carbon can be successfully acidulated using various normalities of HCl where weak solutions can be very effective in functionalizing the surface of the sorbent and capturing mercury. In order to recycle and reuse bamboo derived sorbents, stronger normalities of HCl would be desired. Keywords: Bamboo, Activated Carbon, Mercury, Hydrochloric Acid, Acidulation, Regeneration 1. INTRODUCTION Mercury is a hazardous pollutant present in flue gases fired from coal fired power plants. The toxic effects of mercury are known to cause health concerns in humans through bio- accumulation in the food chain via fish, and also affect other mammals. The US EPA continuously works on developing protocols on the capping of mercury emissions [1].  112 Naved Siddiqui and Jarlen Don Vol.10, No.2 Activated carbons have been reported as sorbent materials numerous times for the capture of elemental mercury (Hg0), which is more difficult to remove than oxidized (Hg2+) and particulate mercury (Hgp) in coal fired plants upstream of particulate control devices including baghouses or electrostatic precipitators (ESP’s) [2, 3, 7]. However, capture of elemental mercury using conventional methods remains elusive due to its low melting point, high vapor pressure, and low solubility in water [3]. Powdered activated carbon injection technology provides very short residence times, mass transfer limitations, and requires a large C/Hg ratio. Therefore, there remains a search for a viable technology for capture of elemental mercury in flue gases of coal- fired plants [3, 4]. Apart from studies conducted on mercury adsorption using charcoal based products such as unburned carbon [5, 6], various studies have been conducted for the production of activated carbons from agricultural by products as reported by [7] such as palm-tree cobs, plum kernels, cassava peel, bagasse, jute fiber, rice husks, olive stones, date pits, fruit stones and nutshells. Bamboo derived activated carbon has been reported as a potential adsorbent several times [7, 8] in aqueous forms. Bamboo, being a material with low sulfur, nitrogen and ash content, and high carbon and oxygen content [10] makes it a very viable material for developing a sorbent for capture of mercury. Also, owing to its high structural integrity, and high growth rate of 30% regeneration in a year [9, 10] make it an attractive choice of using it as a source of activated carbon for adsorption of elemental mercury. Bamboo shows a highly oriented cellular structure, which is a very important property for adsorption characteristics. It belongs to the sub-family Bambusoidae of the family Poaceae (Gramine), which falls under the grass family, and has a typical xylem tissue structure [8, 11]. The first step in order to derive the activated carbon from bamboo is to carbonize it in an inert atmosphere such as nitrogen at temperatures under 1000°C [10, 12], which vaporizes the protoxylem and metaxylem in the bamboo forming large macropores. The carbonizing temperature determines the pore size and surface area of the carbonized bamboo, which according to [12] falls around 490.8 m2g-1 for bamboo charred at 1000°C. Further, the activation process is where the surface porosities are increased, and a large proportion of the xylem is gasified, enlarging the pores, which improves the adsorbing capacity of the carbon. Various methods have been reported for the activation including steaming, where relatively large surface areas have been reported of up to 1038 m2g-1 [8], chemical methods, and also using CO2, with the last method being employed in this study. It has also been reported that activated carbon can only be regenerated a few times before they exhibit unacceptably low activity for mercury removal. It is thought that the mercury removal from a flue gas stream takes place due to a surface functional group (SFG) on the activated carbon. In previous studies, activated carbon was treated with halogens or chalcogens such as Chlorine, Bromine, Iodine and Sulfur which improved the Hg adsorption, and functionalized  Vol.10, No.2 Acidulation and Regeneration of Bamboo Derived Sorbents 113 with O, CO, or OH, or leached with HF to enhance the removal of organic substances in liquids [13, 14, 15, 16]. Also, impregnating virgin Powdered Activated Carbon (PAC) with Cl or S increased the Hg capacity by a few hundred times, e.g., up to 4 mg/g for Hg0 and HgCl2 as compared to virgin carbons which had a capacity ranging from 5 – 50 μg/g [14, 15]. Therefore, chlorine is one such functional group which can be used to functionalize the bamboo derived activated carbon using HCl, as it can improve the Hg0 removal capacity by 300 times as compared to virgin activated carbons [13, 17]. This would also enable the reduction of mineral matter content that would also bring about changes in the surface area and pore texture of the sorbent, since the mineral matter can block a part of the carbon porosity [18]. It is also feasible that this HCl impregnation might be able to recycle or regenerate the activated carbon after it captures the mercury. Other studies have also been conducted where bamboo derived carbon was treated with dilute sulfuric acid for the capture of ammonia in aqueous solutions [19], therefore there is a thought that HCl impregnation could help in functionalizing the surface of bamboo derived sorbents in order to capture mercury in gas phase. The work presented in this study reports a study on activated carbon derived from bamboo in solid form, which have been further functionalized using various normalities of HCl. The performance of elemental mercury uptake under batch tests has been studied. Further batch tests have been conducted on previously used samples, which have again been regenerated using HCl, and tests have been run again. 2. MATERIALS & METHODS 2.1. Preparation of Bamboo Derived Sorbents The first step was to prepare the bamboo derived sorbents, which was done using a series of steps. Raw bamboo slats were acquired from Cali Bamboo and cut into strips of approximately 17.5 cm x 2.2 cm x 0.3 cm in dimensions. In some strips, the outer bark of the bamboo, known as the epidermal tissue was maintained (skin or bark), while in other cases it was shaved off (no- skin or no-bark). This raw material was then processed to develop the bamboo derived sorbent. The first step to prepare any variety of sorbent was carbonization, which was done in order to convert raw bamboo into bamboo charcoal. This process was carried out in a box furnace (CM Rapid Temp Furnace – 1704 series) under continuous flowing nitrogen. The carbonization cycle consisted of a drying time at 200°C for an hour in order to take the moisture out from the bamboo. The samples were then ramped to 800°C at 5°C/min, and then held for 2 hours. The temperature was then allowed to drop naturally. The bamboo strips tended to shrink and bend, and therefore a dead weight was placed on top of the samples to retain the flatness.  114 Naved Siddiqui and Jarlen Don Vol.10, No.2 The carbonized samples were then activated in order to obtain a larger surface area. This process was performed in the box CM Rapid Temp furnace under flowing CO2. They were ramped to 500°C at about 4.2°C/min, and held for 2 hours with a continuous flow at 150 sccm. The temperature was allowed to drop naturally. Application of dead weight was not necessary. The activated samples were acidulated using HCl solutions of various normalities, and allowed to be treated for approximately 3-5 days. This was done on cut samples with desired weights, which was approximately kept at 0.03 grams. These cut samples were washed in about 20 ml distilled water 3 times, and then dried in an oven overnight. Three different HCl solutions were used for acidulation – 0.05N HCl, 0.1N HCl and 1N HCl. In order to recycle or regenerate the samples after adsorption tests were done, a similar procedure was repeated for the Recycling. The 0.1N HCl and 0.05N HCl samples were recycled using a 0.2N HCl solution, and the 1N HCl samples were recycled using a 1N HCl solution. These samples were tested under the Batch Test for mercury adsorption. The surfaces of the developed sorbent materials were characterized through Scanning Electron Microscopy using a Hitachi S570 scanning electron microscope. 2.2. Experimental Setup In order to evaluate the mercury adsorption performance of various prepared sorbents, they were subject to Batch Tests. A schematic of the batch test is shown in Figure 1. Elemental mercury was permeated from a standard mercury permeation tube, acquired from VICI Metronics, which was placed in one section of a glass U-tube. The other section of the U-tube was filled with glass beads. This U-tube was placed in a temperature controlled bath of Corn oil, which was done in a Cole-Parmer – Microprocessor Controlled Water Bath, while using corn oil as the heating medium due to its high flash point. The amount of mercury permeated depended on the temperature of the hot bath and the flow rate of nitrogen through the permeation tube. Transfer- lines, which were primarily Teflon tubing led the gas through the permeation tube into sampling bags, which were acquired from SKC - 231 Series. Two sampling bags were employed, one of which was used as the main sorption chamber (10 L capacity), where the sorbent material was tested, and the other was used as a calibration bag (5L capacity) in order to ensure that the amount of mercury being permeated was correct as desired. The outlet of the sampling bag was connected to the Jerome J405 Mercury Vapor Analyzer, which measured the concentration of mercury contained in the gas in the sampling bag at any given time. The sorbent was loaded into the sampling bag using the small opening in the SKC sampling bag, and then sealed. Prior to each test, the system was flushed with nitrogen at least 3 times in order to make sure there was no oxygen in the system. Once this was done, sorbent material was 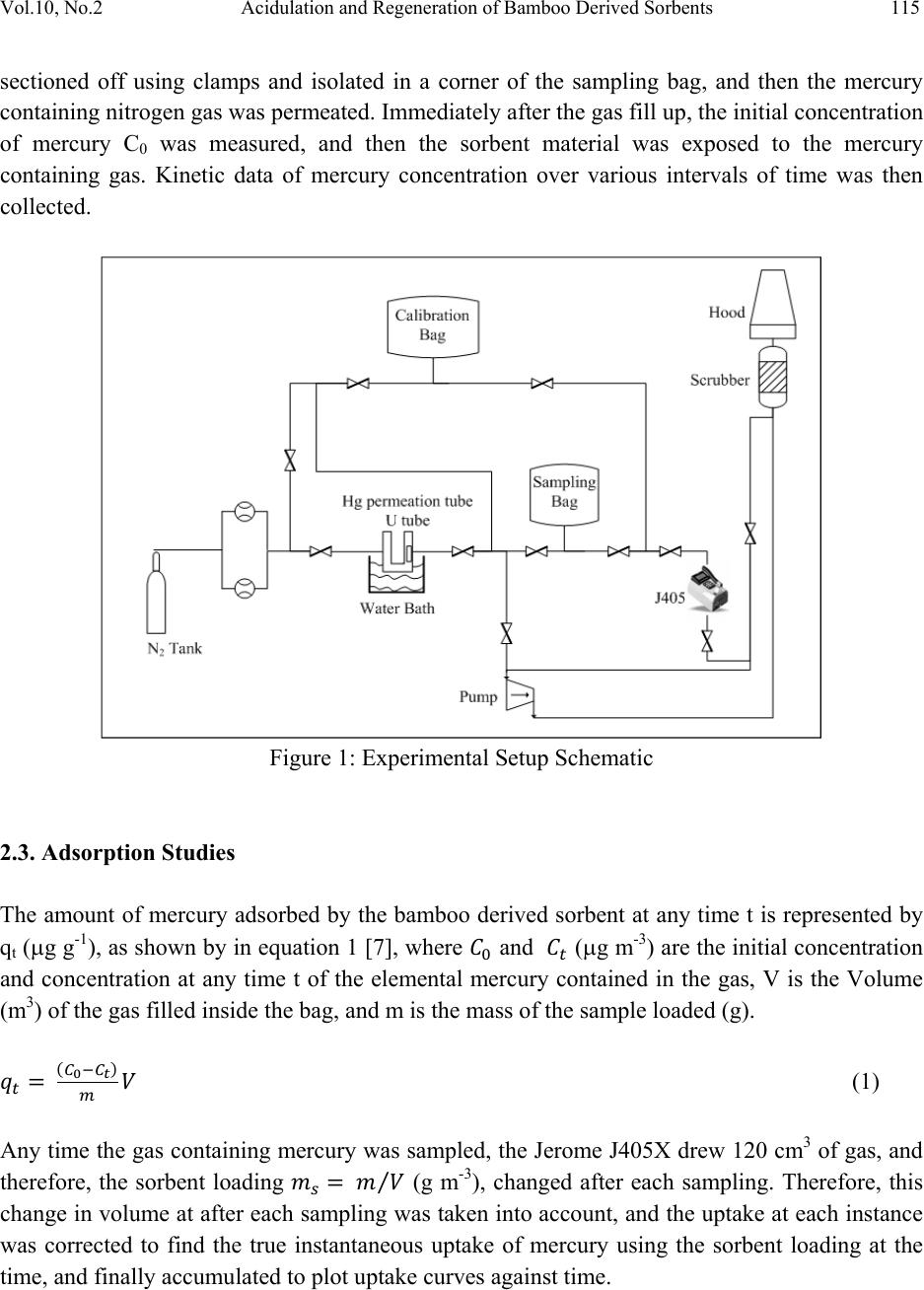 Vol.10, No.2 Acidulation and Regeneration of Bamboo Derived Sorbents 115 sectioned off using clamps and isolated in a corner of the sampling bag, and then the mercury containing nitrogen gas was permeated. Immediately after the gas fill up, the initial concentration of mercury C0 was measured, and then the sorbent material was exposed to the mercury containing gas. Kinetic data of mercury concentration over various intervals of time was then collected. Figure 1: Experimental Setup Schematic 2.3. Adsorption Studies The amount of mercury adsorbed by the bamboo derived sorbent at any time t is represented by qt (g g-1), as shown by in equation 1 [7], where and (g m-3) are the initial concentration and concentration at any time t of the elemental mercury contained in the gas, V is the Volume (m3) of the gas filled inside the bag, and m is the mass of the sample loaded (g). (1) Any time the gas containing mercury was sampled, the Jerome J405X drew 120 cm3 of gas, and therefore, the sorbent loading ⁄ (g m-3), changed after each sampling. Therefore, this change in volume at after each sampling was taken into account, and the uptake at each instance was corrected to find the true instantaneous uptake of mercury using the sorbent loading at the time, and finally accumulated to plot uptake curves against time.  116 Naved Siddiqui and Jarlen Don Vol.10, No.2 First order Kinetics A first order rate equation, developed by Lagergren and reported in many adsorption studies including [20] is described in equation 2 as: (2) where, qe and qt (g/g) describe the adsorption or uptake of elemental mercury by the bamboo derived sorbent at equilibrium and at any time t respectively. k1 describes the overall rate constant of adsorption using the bamboo derived sorbent. Upon integrating and applying the boundary conditions, t = 0 and qt = 0, to t = t and qt = qt, we arrive at ln (3) which can be simplified as 1 (4) Now, if we define a term qm as the maximum uptake of mercury assuming all mercury is adsorbed by the bamboo derived sorbent, then we equation 1 becomes: (5) Dividing equation 1 by equation 4, we can define a term αt, which is essentially a dimensionless normalized uptake of mercury (g m-3/g m-3) at any time t. This αt, presented in equation 5 reduces to 1 (6) Similarly, if we divide equation 4 through by qm, we arrive at a modified form in equation 7 1 (7) where is the normalized equilibrium uptake of mercury Therefore, in order to study the adsorption behavior of each bamboo derived sorbent, the normalized uptake of mercury (g m-3/g m-3) was plotted against time. This behavior was 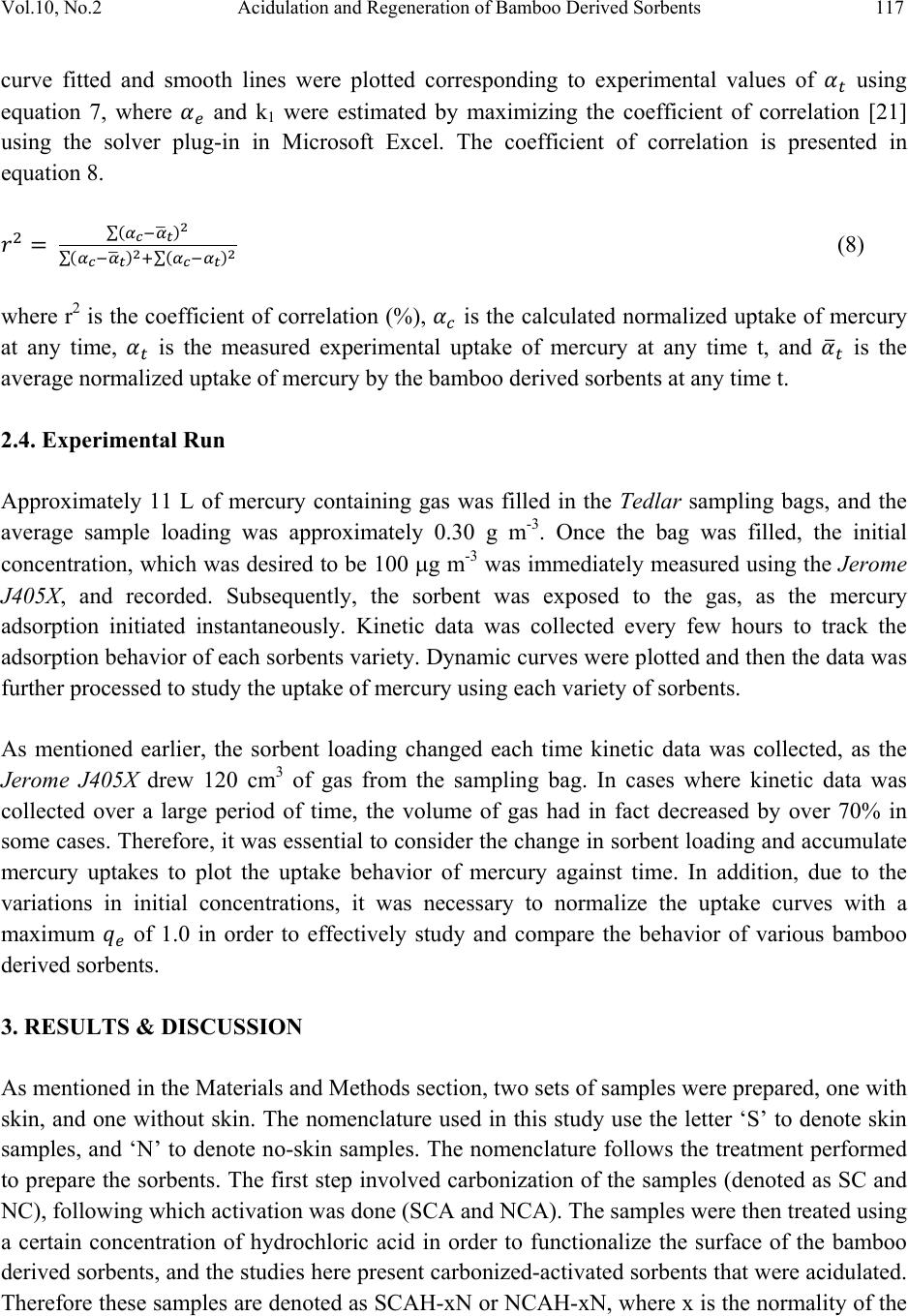 Vol.10, No.2 Acidulation and Regeneration of Bamboo Derived Sorbents 117 curve fitted and smooth lines were plotted corresponding to experimental values of using equation 7, where and k1 were estimated by maximizing the coefficient of correlation [21] using the solver plug-in in Microsoft Excel. The coefficient of correlation is presented in equation 8. ∑ ∑ ∑ (8) where r2 is the coefficient of correlation (%), is the calculated normalized uptake of mercury at any time, is the measured experimental uptake of mercury at any time t, and is the average normalized uptake of mercury by the bamboo derived sorbents at any time t. 2.4. Experimental Run Approximately 11 L of mercury containing gas was filled in the Tedlar sampling bags, and the average sample loading was approximately 0.30 g m-3. Once the bag was filled, the initial concentration, which was desired to be 100 g m-3 was immediately measured using the Jerome J405X, and recorded. Subsequently, the sorbent was exposed to the gas, as the mercury adsorption initiated instantaneously. Kinetic data was collected every few hours to track the adsorption behavior of each sorbents variety. Dynamic curves were plotted and then the data was further processed to study the uptake of mercury using each variety of sorbents. As mentioned earlier, the sorbent loading changed each time kinetic data was collected, as the Jerome J405X drew 120 cm3 of gas from the sampling bag. In cases where kinetic data was collected over a large period of time, the volume of gas had in fact decreased by over 70% in some cases. Therefore, it was essential to consider the change in sorbent loading and accumulate mercury uptakes to plot the uptake behavior of mercury against time. In addition, due to the variations in initial concentrations, it was necessary to normalize the uptake curves with a maximum of 1.0 in order to effectively study and compare the behavior of various bamboo derived sorbents. 3. RESULTS & DISCUSSION As mentioned in the Materials and Methods section, two sets of samples were prepared, one with skin, and one without skin. The nomenclature used in this study use the letter ‘S’ to denote skin samples, and ‘N’ to denote no-skin samples. The nomenclature follows the treatment performed to prepare the sorbents. The first step involved carbonization of the samples (denoted as SC and NC), following which activation was done (SCA and NCA). The samples were then treated using a certain concentration of hydrochloric acid in order to functionalize the surface of the bamboo derived sorbents, and the studies here present carbonized-activated sorbents that were acidulated. Therefore these samples are denoted as SCAH-xN or NCAH-xN, where x is the normality of the  118 Naved Siddiqui and Jarlen Don Vol.10, No.2 hydrochloric acid solution. An attempt was made to regenerate acidulated samples, where after a batch-test; they were retreated with a certain HCl solution. Therefore, these samples are termed as SCAH-xN-RxN or NCAH-xN-RxN. An analysis of these samples is presented in the following paragraphs. In general, it was found that each subsequent treatment for the preparation of the bamboo derived sorbent improved the uptake performance of the bamboo derived sorbents, in the increasing order of carbonization, carbonization-activation, carbonization-activation-acidulation. The carbonized-activated-regenerated-acidulated samples were retreated with a certain concentration of hydrochloric acid, and it was desired to match initial performance. The overall conditions including mass of sorbent loaded (g), initial concentration C0, and overall rate constants are presented in Table 1. These are discussed corresponding to the uptake behavior of each sorbent as presented in the following figures and paragraphs. Based on the adsorption kinetics presented in the previous section, and observing the graphs, we find fairly good fits for mercury uptake using first order kinetics. This can be verified using the coefficient of correlation, which is also provided in Table 1, which gives us an idea of the accuracy of overall fits. Table 1: Overall results describing the uptake behavior of various bamboo derived sorbents mass (g)C0 ( g m-3)k1 (h-1)R 2 Skin Samples SC 0.0278 103.97 0.0072 0.9750 SCA 0.0277 98.30 0.0224 0.9955 SCAH1N 0.0277 96.97 0.0365 0.9980 SCAH1N-R1N 0.0276 94.67 0.0329 0.9983 No Skin Samples NC 0.0278 93.00 0.0053 0.9946 NCA 0.0270 122.30 0.0178 0.9618 NCAH0.05N 0.0272 116.05 0.0477 0.9908 NCAH0.05N - R0.2N0.0262 96.17 0.0245 0.9993 NCAH0.1N 0.0274 110.60 0.0489 0.9903 NCAH0.1N - R0.2N 0.0267 108.00 0.0224 0.9976 NCAH1N 0.0283 98.62 0.0557 0.9996 NCAH1N-R1N 0.0279 94.08 0.0859 0.9868 Figure 2 primarily presents the uptake of mercury using sorbents of various treatments where the epidermal tissue was kept intact. It can clearly be seen from these curves that the adsorption performance of the bamboo derived sorbents improved considerable with each subsequent performance. The overall rate constants of the Skin-Carbonized was found to be 0.0072 h-1. 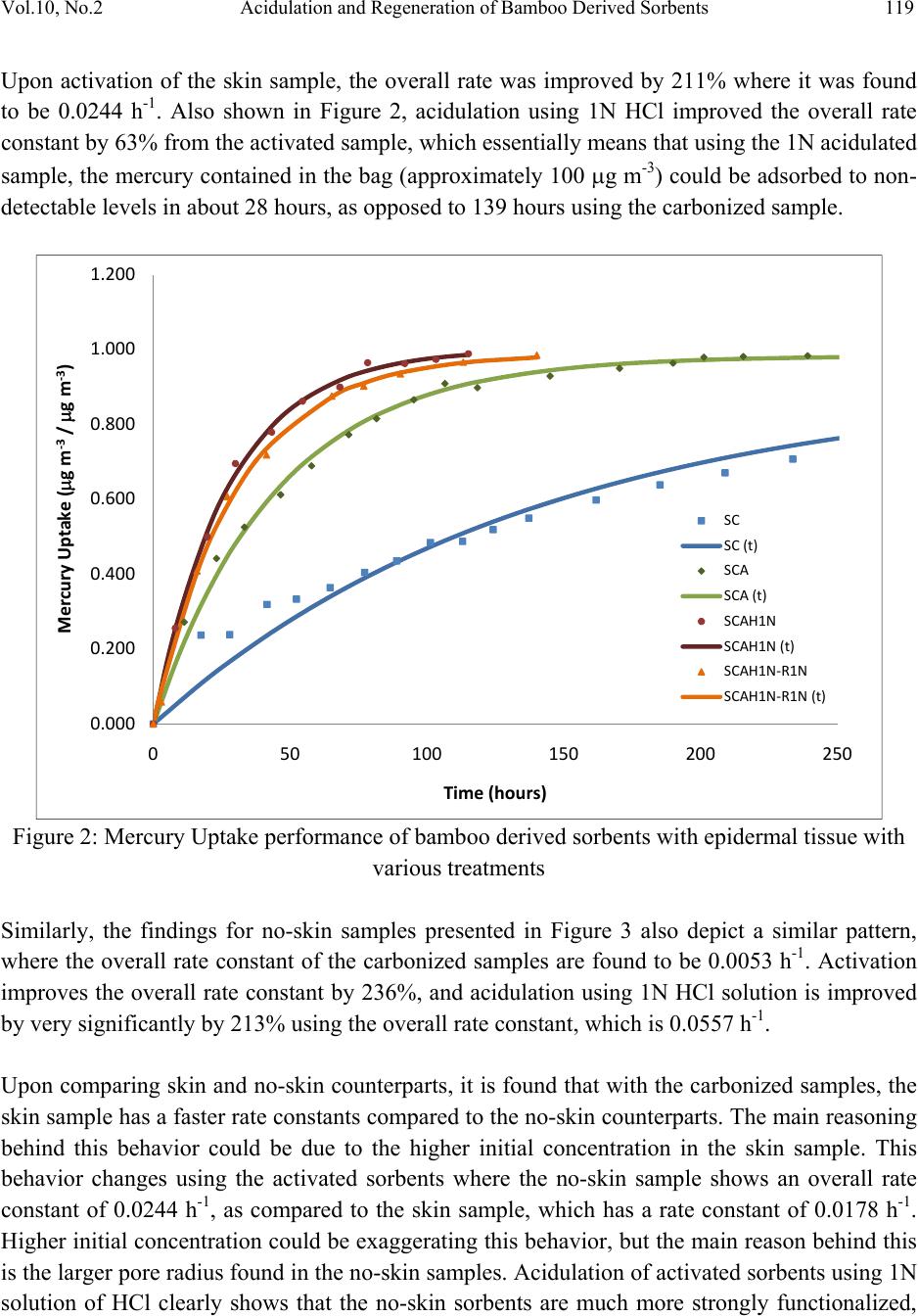 Vol.10, No.2 Acidulation and Regeneration of Bamboo Derived Sorbents 119 Upon activation of the skin sample, the overall rate was improved by 211% where it was found to be 0.0244 h-1. Also shown in Figure 2, acidulation using 1N HCl improved the overall rate constant by 63% from the activated sample, which essentially means that using the 1N acidulated sample, the mercury contained in the bag (approximately 100 g m-3) could be adsorbed to non- detectable levels in about 28 hours, as opposed to 139 hours using the carbonized sample. Figure 2: Mercury Uptake performance of bamboo derived sorbents with epidermal tissue with various treatments Similarly, the findings for no-skin samples presented in Figure 3 also depict a similar pattern, where the overall rate constant of the carbonized samples are found to be 0.0053 h-1. Activation improves the overall rate constant by 236%, and acidulation using 1N HCl solution is improved by very significantly by 213% using the overall rate constant, which is 0.0557 h-1. Upon comparing skin and no-skin counterparts, it is found that with the carbonized samples, the skin sample has a faster rate constants compared to the no-skin counterparts. The main reasoning behind this behavior could be due to the higher initial concentration in the skin sample. This behavior changes using the activated sorbents where the no-skin sample shows an overall rate constant of 0.0244 h-1, as compared to the skin sample, which has a rate constant of 0.0178 h-1. Higher initial concentration could be exaggerating this behavior, but the main reason behind this is the larger pore radius found in the no-skin samples. Acidulation of activated sorbents using 1N solution of HCl clearly shows that the no-skin sorbents are much more strongly functionalized, 0.000 0.200 0.400 0.600 0.800 1.000 1.200 050100 150 200 250 MercuryUptake(gm‐3/gm‐3) Time(hours) SC SC(t) SCA SCA(t) SCAH1N SCAH1N(t) SCAH1N‐R1N SCAH1N‐R1N(t)  120 Naved Siddiqui and Jarlen Don Vol.10, No.2 and show far greater adsorption performance with an overall rate constant 52.60% greater at of 0.0557 h-1 as compared to 0.0365 h-1. A comparison between skin and no-skin sample is also shown in SEM studies following the quantitative discussion. Figure 3: Mercury Uptake performance of bamboo derived sorbents without epidermal tissue with various treatments As it was determined that acidulation using no-skin samples provided much stronger functionalization on the surface of the bamboo derived sorbents, an effort was made to vary the concentrations of HCl solutions and study the behavior of mercury uptakes using these samples. 0.05N HCl functionalization improved the performance activated no skin sample by 168%, whereas 0.1N HCl functionalization improved it by 175%, with overall rate constants of 0.0477 and 0.0489 g g-1 h -1 respectively. The 1N HCl solution improved the performance from activated samples by 213%, which is not drastically different compared to the weaker acidulated solutions. Therefore, even a weak solution of HCl can be very effective in improving the adsorption performance of the bamboo derived sorbents. Further, the regeneration of bamboo derived sorbents was addressed. Samples that had undergone a batch test to adsorbed mercury were collected and re-treated using a certain solution of HCl, and tested over. The samples that were originally acidulated using 1N solution of HCl were regenerated using a fresh 1N solution of HCl, whereas the samples originally treated using 0.05N or 0.1N solution of HCl were regenerated using 0.02N solution of HCl. The goal was to 0.00 0.20 0.40 0.60 0.80 1.00 1.20 050100 150 200 250 MercuryUptake(gm‐3/gm‐3) Time(hours) NC NC(t) NCA NCA(t) NCAH1N SCAH1N(t) NCAH1N‐R1N NCAH1N‐R1N(t)  Vol.10, No.2 Acidulation and Regeneration of Bamboo Derived Sorbents 121 reproduce the performance of original samples. It can be seen in Figure 4 that neither of the two samples that were regenerated using 0.02N solution of HCl were able to match the performance of original samples. The sample NCAH0.05N having an overall rate constant of 0.0477 h-1 was much faster than regenerated sample, which was labeled as NCAH0.05N-R0.2N and had a rate constant of 0.0245 h-1. In other words, the rate constant had decreased by 48.64%. A similar finding was seen when the 0.1N HCl acidulated sample, labeled as NCAH0.1N having an overall rate constant of 0.0489 h-1 was regenerated to NCAH0.1N-R0.2N, which had a rate constant of 0.0245 h-1 implying that this sample was 49.90% slower. This indicated that the regeneration of a sample needs to be done with a rather stronger solution of HCl. Figure 4: Regeneration of acidulated bamboo derived sorbents without epidermal tissue using low HCl concentrations These cases have been shown in Figures 2 and 3, where the 1N treated samples SCAH1N and NCAH1N, having overall rate constants of 0.0365 and 0.0557 h-1 respectively were regenerated using a fresh 1N solution of HCl. The regenerated sample SCAH1N-R1N had an overall rate constant of 0.0329 g g-1 h -1, which showed significant regeneration, as the sample was only 9.86% slower than the original run. The sample NCAH1N-R1N had an overall rate constant of 0.0859 g g-1 h -1, implying that the mercury adsorption performance of this sample had improved by 54.21%. In fact the initial rate indicates that this is 85.88% better. This goes on to explain that the bamboo derived sorbents can not only be successfully regenerated using strong acidulations of HCl, but also that there is a strong indication that more vigorous treatment of 0.000 0.200 0.400 0.600 0.800 1.000 1.200 050100 150 200 250 MercuryUptake(gm‐3/gm‐3) Time(hours) NCAH0.05N NCAH0.0.5N(t) NCAH0.05N‐R0.2N NCAH0.05N‐R0.2N(t) NCAH0.1N NCAH0.1N(t) NCAH0.1N‐R0.2N NCAH0.1N‐R0.2N(t)  122 Naved Siddiqui and Jarlen Don Vol.10, No.2 acidulation can drastically improve the adsorption performance of these bamboo derived sorbents, which can be very useful in real life situations in coal-fired plants. The SEM imaging conducted (Figure 5 and 6) illustrates the difference between bamboo derived sorbents where the epidermal tissue was kept intact (skin) and a sample where the epidermal tissue was removed (no-skin). It can clearly be seen that upon carbonization of the two samples under the same conditions, there is a much greater concentration of the protoxylem and metaxylem in the skin samples as opposed to the no skin samples, which is evidence of gasification of stronger gasification of xylem tissue. The bark material is excellent in providing structural integrity, which is important from a practical standpoint, however, based on performance, the no skin sample, i.e. without the bark material provides more gasified xylem tissues, which lead to micro-porosities through the channels in the bamboo based structure. Similar findings were illustrated when the samples were activated, where the xylem tissue was further gasified, and there is a strong correlation with experimental data, which shows that the NCA samples provides far better adsorption performance as compared to SCA samples. Figure 5: Carbonized-Skin SEM Figure 6: Carbonized-No Skin SEM As an overall conclusion, which can be illustrated by looking at rate constants in the bar charts provided in Figures 7 and 8, illustrate the improvement in performance of bamboo derived  Vol.10, No.2 Acidulation and Regeneration of Bamboo Derived Sorbents 123 sorbents upon each subsequent treatment of carbonization, activation, and further; acidulation. Also addressed was the recycling of HCl functionalized bamboo derived sorbents. It can be seen that the bamboo derived sorbents that were treated with the weaker 0.1N HCl and 0.05 N HCl solutions showed very good mercury uptake performance in original runs. However, when attempted to be recycled using a 0.02N HCl solution, the sorbents did not match original performance. Conversely when the bamboo derived activated carbon was acidulated using 1N solution of HCl, and subsequently recycled using a fresh 1N solution, not only was the original mercury uptake performance matched, but it was surpassed. This went on to prove the hypothesis that hydrochloric acid treatment is a very effective way of elemental mercury capture in gas phase. Originally, a weak acid solution could be enough to funtionalize the surface of an activated bamboo derived sorbent for enhanced mercury capture, which could be regenerated using a stronger than original treatment solution. This could be a very effective way of retrofitting an effective material in flue streams of coal fired plants for mercury capture. Figure 7: Rate Constants for mercury uptake by bamboo derived sorbents with skin with various treatments 0.0000 0.0200 0.0400 0.0600 0.0800 0.1000 SCSCASCAH1N SCAH1N‐R1N  124 Naved Siddiqui and Jarlen Don Vol.10, No.2 Figure 8: Rate Constants for mercury uptake by bamboo derived sorbents with skin with various treatments ACKNOWLEDGEMENTS This research was made possible with support, in part from the Illinois Department of Commerce and Economic Opportunity through the Office of Coal Development and the Illinois Clean Coal Institute (Grant No. ICCI 07-1). REFERENCES 1. EPA Mercury Home, online: http://epa.gov/mercury/ [August 5th 2010] 2. Jones, A., Hoffman, J., Smith, D., Feeley III, T., and Murphy. J., 2007, “DOE/NETL’s Phase II Mercury Control Technology Field Testing Program: Preliminary Economic Analysis of Activated Carbon Injection.” Environ. Sci. Technol., Vol. 41, Issue 4, pp. 1365-1371. 3. His, H., Chen, S., Rostam-Abadi, M., Rood, M., Richardson, C., Carey, T., and Chang, R., 1998, “Preparation and Evaluation of Coal-Derived Activated Carbons for Removal of Mercury Vapor from Simulated Coal Combustion Flue Gases”, Energy & Fuels, Vol. 12, Issue 6, pp. 1061-1070 4. Bustard, J., Durham, M., Starnes, T., Lindsey, C., Martin, C., Schlager, R., and Baldrey K, 2004, “Full-Scale evaluation of sorbent injection for mercury control on coal-fired power plants”, Fuel Proces. Technol. Vol. 85, Issue 6-7, pp. 549-562 0.0000 0.0200 0.0400 0.0600 0.0800 0.1000  Vol.10, No.2 Acidulation and Regeneration of Bamboo Derived Sorbents 125 5. Li, Z., Sun, X., Luo, J., Hwang J., and Crittenden J., 2002, “Unburned Carbon from Fly Ash for Mercury Adsorption: II. Adsorption Isotherms and Mechanisms”, Journal of Minerals & Materials Characterization & Engineering, Vol. 1, No. 2, pp. 79-96 6. Luo, J., Hein, A., and Hwang, J., 2004 “Adsorption of Vapor Phase Mercury on Various Carbons”, Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No. 1, pp. 13-22 7. Hameed, B., Din, A., and Ahmad, A., 2007, “Adsorption of methylene blue onto bamboo- based activated carbon: Kinetics and equilibrium studies”, Journal of Hazardous Materials, Vol. 141, Issue 3, pp. 819-825 8. Wu, F., Tseng, R., and Juang, R., 1999, “Preparation of activated carbons from bamboo and their adsorption abilities for dyes and phenols”, J. Environ. Sci. Health, Vol. A34, Issue 9, pp. 1753-1775. 9. Granite, E., Pennline, H., Hargis, R., 2000, “Novel Sorbents for Mercury Removal from Flue Gas”, Ind. Eng. Chem. Res., Vol. 39, Issue 4, pp. 1020-1029. 10. Mui, E., Cheung, W., Lee, V., and McKay, G., 2008, “Kinetic Study on Bamboo Pyrolysis”, Ind. Eng. Chem. Res., Vol. 47, Issue 15, pp. 5710-5722 . 11. Scurlock, J., Dayton, D., and Hames, B., 2000, “Bamboo: an overlooked biomass resource”, Biomass & Bioenergy, Vol. 19, Issue 4, pp. 229- 244. 12. Asada, T., Ishihara, S., Yamane, T., Toba, A., Yamada, A., Oikawa, K., 2002, “Science of Bamboo Charcoal : Study on Carbonizing Temperature of Bamboo Charcoal and Removal Capability of Harmful Gases”, Journal of H ealth Science, Vol. 48, Issue 6, pp. 473-479. 13. Ghorishi, S., Keeney, R., Serre, S., and Gullett, B., 2002, “Development of a Cl-Impregnated Activated Carbon for Entrained-Flow Capture of Elemental Mercury”, Environ. Sci. Technol., Vol. 36, Issue 20, pp. 4454-4459. 14. Ghorishi, S. B.; Gullett, B. K., 1997, “Fixed-bed control of mercury; role of acid gases and a comparison between carbon-based, calcium-based, and coal fly ash sorbents”, Presented at the 1st EPRI-DOE/EPA Combined Utility Air Pollutant Control Symposium, Washington, DC. 15. Qu, Z., Yan, N., Liu, P., Chi, Y., and Jia, J., 2009, “Bromine Chloride as an Oxidant to Improve Elemental Mercury Removal from Coal-Fired Flue Gas”, Environ. Sci. Technol., Vol. 43, Issue 22, pp. 8610-8615. 16. Luo, J., Hwang, J., Greenlung, B., Sun, X., and Xu, Z., 2004, “Adsorption of Hg0 on the Unburned Carbon with HF Acid Leaching”, Journal of Minerals & Materials Characterization & Engineering, Vol. 3, No. 1, pp. 41-51 17. Liu, W., Vidic R., and Brown, T., 2000, “Impact of flue conditions on Mercury uptake by Sulfur-Impregnated Activated Carbon, Environ. Sci. Techn ol.”, Vol. 34, Issue 1, pp. 154-159. 18. Moreno-Castilla, C., Carrasco-Marin, F., Maldonado-Hodar F., Riviera-Utrilla, J., 1998, “Effects of Non-Oxidant and Oxidant Acid Treatments on the Surface Properties of an Activated Carbon with Very Low Ash Content”, Carbon, 36, Issue 1-2, pp. 145-151.  126 Naved Siddiqui and Jarlen Don Vol.10, No.2 19. Asada, T., Ohkubo, T., Kawata, K., and Oikawa, K., 2006, “Ammonia Adsorption on Bamboo Charcoal with Acid Treatment”, Journal of Health Science, Vol. 52, Issue 5, pp. 585-589. 20. Igwe, J., Abia, A., and Ibeh, C., 2008, “Adsorption kinetics and intraparticulate diffusivities of Hg, As and Pb ions on unmodified and thiolated coconut fiber”, Int. J. Environ. Sci. Tech., Vol. 5, Issue 1, pp. 83-92. 21. Ho, Y., 2006, “Second order kinetic model for the sorption of cadmium ions onto tree fern: A comparison of linear and non-linear methods”, Water Research, Vol. 40, Issue 1, pp. 119- 125. |

