 J. Biomedical Science and Engineering, 2010, 3, 227-240 doi:10.4236/jbise.2010.33031 Published Online March 2010 (http://www.SciRP.org/journal/jbise/ JBiSE ). Published Online March 2010 in SciRes. http://www.scirp.org/journal/jbise Quantifying the effects of mutations on receptor binding specificity of influenza viruses Wei Hu Department of Computer Science, Houghton College, Houghton, USA. Email: wei.hu@houghton.edu Received 7 January 2010; revised 11 January 2010; accepted 15 January 2010. ABSTRACT Hemagglutinin (HA) of influenza viruses is a cylin- drically shaped homotrimer, where each monomer comprises two disulfide-linked subdomains HA1 and HA2. Influenza infection is initiated by binding of HA1 to its host cell receptors and followed by the fusion between viral and host endosomal membranes mediated by HA2. Human influenza viruses prefer- entially bind to sialic acid that is linked to galactose by an α2,6-linkage (α2,6), whereas avian and swine influenza viruses preferentially recognize α2,3 or α 2,3/α2,6. For animal influenza viruses to cross host species barriers, their HA proteins must acquire mu- tations to gain the capacity to allow human-to-human transmission. In this study, the informational spec- trum method (ISM), a bioinformatics approach, was applied to identify mutations and to elucidate the contribution to the receptor binding specificity from each mutation in HA1 in various subtypes within or between hosts, including 2009 human H1N1, avian H5N1, human H5N1, avian H1N1, and swine H1N2. Among others, our quantitative analysis indicated that the mutations in HA1 of 2009 human H1N1 col- lectively tended to reduce the swine binding affinity in the seasonal H1N1 strains and to increase that in the pandemic H1N1 strains. At the same time, they increased the human binding affinity in the pandemic H1N1 strains and had little impact on that in the sea- sonal H1N1 strains. The mutations between the con- sensus HA1 sequences of human H5N1 and avian H5N1 increased the avian binding affinity and de- creased the human binding affinity in avian H5N1 while produced the opposite effects on those in hu- man H5N1. Finally, the ISM was employed to analyze and verify several mutations in HA1 well known for their critical roles in binding specificity switch, in- cluding E190D/G225D in H1N1 and Q192R/ S223L/ Q226L/ G228S in H5N1. Keywords: Binding Specificity; Discrete Fourier Transform; Electron-Ion Interaction Potential; Entropy; Hemagglutinin; Influenza; Informational Spectrum Method; Mutation; Receptor 1. INTRODUCTION Influenza A viruses are classified into different sub- types based on the viral surface proteins hemagglutinin (HA) and neuraminidase (NA). The initial step in the influenza infection is the binding of HA to sialylated glycan receptors on the host cells. HA is also the pri- mary target for the immune response in the infected host. Human and swine influenza viruses are derived from avian viruses, facilitated by regular close contact among humans, birds, and pigs [1]. The past three in- fluenza pandemics, the Spanish flu (H1N1) in 1918, the Asian flu (H2N2) in 1957, and the Hong Kong flu (H3N2) in 1968, all had arisen from a reassortment from avian, swine, and human viruses. The current 2009 influenza pandemic was caused by a swine-origin H1N1 virus. The adaptation of the virus to a new host entails the compatibility between host and virus genetic requirements to allow efficient replication and sus- tained transmission. The host barrier for influenza vi- ruses to transmit in humans is multigenic, however, the receptor specificity of HA proteins is a key determinant. The binding preference of influenza virus HAs af- fects the host specificity for infection. In general, hu- man influenza viruses bind preferentially to α2,6 re- ceptors, avian influenza viruses tend to bind to α2,3 receptors, and swine influenza viruses can bind to ei- ther α2,6 receptors or both α2,6 and α2,3 receptors, primarily based on differences in the amino acids in the HA receptor binding domain (RBD). The RBD of HA in various influenza subtypes has three structural elements in common, one α-helix (190-helix) and two loops (130-loop and 220-loop). Different hosts express diverse SA isomers, i.e., α2,3 linkages in the gut of waterfowl, α2,3 and α2,6 linkages in the lung and in- testinal epithelium of chickens, and α2,6 linkages in the upper respiratory epithelium and α2,3 and α2,6 link- ages in the lower respiratory epithelium of humans [2]. A change of binding preference is essential for cross species transfer, which involves mutations in HA to alter its glycan receptor preference [3]. It is hypothesized that  228 W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. JBiSE to facilitate efficient human transmission, mutations in HA are required to increase α2,5 binding and at the same time to decrease α2,3 binding [4]. A variety of mutations that can shift receptor preference of HA proteins have been identified. In a study of receptor specificity of influenza A/H5 vi- ruses [5], all but two isolates exhibited high affinity to α2,3 receptors. The two isolates with a unique S223N change in HA demonstrated decreased affinity to α2,3 and moderate affinity to α2,6 receptors. Another study showed that introduction of the mutation Q192R enhanced the binding of HA in H5N1 to α2,6 receptors, and introduc- tion of both mutations Q192R and S223N increased the binding preference significantly. Residue 192 is close to the 190 helix, and residue 223 is part of the 220 loop, where it is feasible for them to influence the binding affin- ity [6]. As for the H2, H3, H4, and H9 HAs, two substitu- tions Q226L and G228S are mainly responsible for the switch from avian to human binding [3,7,8,9]. Residues 138, 186, 190, 194, 225, 226, and 228 can modulate the binding affinity of H1N1 HA proteins, and two residues 190 and 225 play key dominant roles in binding affinity [10,11]. The sequences of the RBD of avian H1 viruses maintain a Glu at position 190 and a Gly at 225 (H3 numbering), while the human H1 viruses gen- erally have an Asp at both positions 190 and 225. It is known that E190D and G225D mutations in H1 viruses can shift binding patterns from avian to human type. In the five 1918 H1N1 HA sequences, three have a D190 and a D225 with α2,6 affinity, and two have a D190 and a G225 with mixed α2,6/α2,3 specificities [12]. In general, mutations D190/D225 favor α2,6 receptors in humans, D190/G225 like α2,6 and α2,3 receptors in swine, and E190/G225 prefer α2,3 receptors in avian [13]. The bio- chemical analysis in [14] quantified the multivalent HA-glycan interactions, and showed the effects of these mutations on glycan binding amplified by multivalency. To date, the symptoms of 2009 H1N1 are mild. The fear is that the virus may continue to mutate to bring about another more lethal outbreak in the subsequent months as the 1918 Spanish flu. In [15] two representa- tive 2009 H1N1 HA sequences, A/California/4/2009 and A/Hamburg/5/2009, were shown to bind to both α2,6 and α2,3 receptors with some minor differences in a carbohydrate microarray analysis, as predicted in [16]. There were three amino acid mutations between these HA sequences: S83P, A197T, and V321I, which might account for these differences in binding. These findings suggested that no major change in binding affinity is necessary for pandemic virus to acquire human binding patterns, and the dual binding to α2,6/α2,3 receptors is one contributor to the greater virulence of the pandemic virus than seasonal flu virus. There were two other recent reports on the mutations of the 2009 H1N1 virus. The first report [17] located the potential mutations and strongly co-mutated positions in NA. The second report [18] focused on HA and the inter- action between HA and NA. The mutations of HA in 2009 H1N1 were found and mapped to the 3D homology model of H1, and the mutations on the five epitope regions on H1 were identified. With help from the results of the first study, two co-mutation networks were uncovered, one in HA and one in NA, where each mutation in one network co-mutates with the mutations in the other network across the two proteins HA and NA. These two networks resid- ing in HA and NA separately may provide a functional linkage between the mutations that can change the drug binding sites in NA and those that can affect the host im- mune response or vaccine efficacy in HA. In references [19,20] the informational spectrum method (ISM) [21] was applied to investigate the inter- action between HA and its receptors, which showed that HA1 of different flu subtypes encodes one highly con- served domain that might be determinants of HA binding affinity. The study in [22] extended the results in refer- ences [19,20] by identifying multiple domains in HA1 associated with each receptor interaction pattern. These conserved domains in HA1 might be used to identify new therapeutic targets for drug development. In references [19,20] it was found that the consensus informational spectrum (CIS) of HA1 of influenza strains have the following characteristic dominant peaks at dif- ferent IS frequencies as presented in Table 1. In this study, F(0.295) will be referred to as pandemic human H1N1 receptor interaction frequency, F(0.055) as swine receptor interaction frequency, F(0.076) as avian receptor interac- tion frequency, and F(0.236) as seasonal human H1N1 receptor interaction frequency. In addition to the dominant peak at IS frequencies in each subtype, there are secon- dary peaks at various IS frequencies [19,20,22]. Viral evolution can help influenza viruses surmount species barriers. Once adapted in a new host, they still need to continue their evolution to fit better in the new environment. In this study, we sought to investigate the effects of mutations in HA1, either within or between hosts, on binding preference shift through a quantitative analysis, the ISM. The analysis performed in this study was based on the observation that several influenza vi- ruses display dual specific recognition of receptors with α2,6 or α2,3 linkages. Our goal was to utilize the ISM to uncover the amino acid polymorphisms in HA1 within or between hosts and to measure their contribution to the binding specificity switch quantitatively. Table 1. Characteristic IS frequencies of HA proteins in 2009 H1N1, swine H1N1/H1N2, avian H5N1, and seasonal human H1N1. Subtype 2009 H1N1 Swine H1N2/H1N1 Avian H5N1 Seasonal human H1N1 FrequencyF(0.295) F(0.055) F(0.076) F(0.236)  W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. 229 2. MATERIALS AND METHODS 2.3. Important Sites in HA Although there is a great variation due to high selection pressure in the HA1 sequences of various flu subtypes, the active site of HA1 is well conserved, which is lo- cated in a cleft composed of the residues 91, 150, 152, 180, 187, 191, and 192. The three amino acids at posi- tions 187, 191 and 192 are a part of the 190 helix. The active site cleft of HA is formed by its right edge (131_GVTAA) and left edge (221_RGQAGR) (H1 num- bering), which are also commonly referred to as the 130 loop and 220 loop, respectively [25,26]. 2.1. Sequence Data All HA sequences were retrieved from the Influenza Virus Resource (http://www.n cbi/nlm.nih.giv/gen omes/ FLU/FLU.html) of the National Center for Biotechnol- ogy Information (NCBI) on November 20, 2009. Only the full length and unique sequences were selected. There were 450 HA sequences of human 2009 H1N1, 201 HA sequences of human H5N1 from 1979 to 2009, 1228 HA sequences of avian H5N1 from 1959 to 2009, 78 HA sequences of avian H1N1 from 1976 to 2008, and 83 HA sequences of swine H1N2 from 1980 to 2009. All the sequences used in the study were aligned with MAFFT [23]. 3. RESULTS 3.1. Mutations within Hosts 3.1.1 2009 Human H1N1 2.2. Entropy After visual inspection of the alignment of 2009 human H1N1 HA1 sequences, there was either an Asp (single letter code D) at position 127 in the pandemic strains or a deletion at position 127 in the seasonal strains. Since Asp had the highest EIIP value of 0.128 [22], this dele- tion at position 127 might influence the DFT spectral distribution. Of the 450 HA1 sequences of 2009 human H1N1 collected, there were 345 pandemic H1N1 se- quences with an Asp at position 127 and 105 seasonal H1N1 sequences with a deletion at position 127. In information theory [24], entropy is a measure of dis- order or randomness associated with a random variable. Let be a discrete random variable that has a set of possible values with probabilities where n aaaa ,...,, 221 i axP i p n pp ,... 1p3 p,, 2 . The entropy H of is ii pxH log i p In the current study, each of the n columns in a multi- ple sequence alignment of a set of HA sequences of N residues is considered as a discrete random variable (1 ≤i ≤N) that takes on one of the 20 (n=20) amino acid types with some probability. has its minimum value 0 if all the residues at position i are the same, and achieves its maximum if all the 20 amino acid types ap- pear with equal probability at position i, which can be i x i xH The CIS of the pandemic H1N1 HA1 sequences were plotted in Figure 1(a), which have a dominant peak at frequency F(0.295) (pandemic human H1N1 binding), and the CIS of the seasonal H1N1 HA1 sequences were plotted in Figure 1(b), which have a dominant peak at frequency F(0.055) (swine binding). According to the ISM concepts, this demonstrated the different receptor binding patterns of the 2009 pandemic and seasonal H1N1 strains. Figure 2 illustrated the consensus se- quences of the pandemic H1N1 strains (human binding preference) and seasonal H1N1 strains (swine binding preference), respectively. verified by the Lagrange multiplier technique. A position of high entropy means that the amino acids are often varied at this position. measures the genetic di- versity at position i in our current study. A brief over- view of the extensive applications of entropy in se- quence analysis, in particular the flu virus sequences, can be found in [17]. i xH There were 90 amino acid substitutions between the two consensus HA1 sequences of human and swine binding characteristics (Figure 2 and Table 2). Based on Table 2. Mutations between swine and human bindings in HA1 sequences of 2009 H1N1. I3L N35D S36K L43K K45R I47V Q51H N54KS56NV57IL69SI71SS72TK73A E74S K82T P83SN84S P85S E86D H94D A96I E120T -127D S128T V129NT130KS133TS135AS137PN139AE141A S142K R146K L149IT152V G153K N155G G156N L157S N160K A166I N167D E170G V179IP183SN184TI185SV186A K189Q T190S H193Q T194NE195A N196D S200F V202G H205R R208K T211K K216I I227ML234V T239KI241TN245TI249VA250V L257M S258E G260NF261A N267I N269D A270T M272V D273H K274D D276N A277TK278T Q283KV295IV298IE302K R308K A310T M314L V315AI321V JBiSE  230 W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. ( a) (b) (c) (d) Figure 1. (a) CIS of HA1 of 200 CIS of HA1 of 2009 ); e ISM theory, the mutations in HA1 that increased the binding pocket, which were not listed in Table 3 because dues 269, 00.05 0.10.15 0.20.25 0.30.350.40.450.5 0 50 100 150 200 250 300 CIS of HA1 of 2009 H1N1 (swine binding) Frequency S/N 00.05 0.10.15 0.20.25 0.3 0.35 0.4 0.45 0.5 0 50 100 150 200 250 300 CIS of HA1 of 2009 H1N1 (pandemic human H1N1 binding) Frequency S/N F(0.055) F(0.295) JBiSE 9 pandemic H1N1 (pandemic human H1N1 binding); (b) seasonal H1N1 (swine H1N1 binding); (c) IS of HA1 of 2009 pandemic H1N1 (pandemic human H1N1 binding (d) IS of HA1 of 2009 seasonal H1N1 (swine H1N1 binding). th amplitude of F(0.295) and decreased that of F(0.055) would contribute the switch of receptor binding affinity from swine to human type. The variation amount of the amplitudes of F(0.295) and F(0.055) was calculated for each of the 90 mutations applied to each consensus HA1 sequence, swine binding or human binding. The top 32 mutations that resulted in the amplitude change at fre- quency F(0.295) or F(0.055) (∆A) more than 6% were listed in Table 3, suggesting that these mutations might be critical for modulating the binding preferences be- tween swine and humans. In general, increasing the am- plitude at one frequency F(0.295) or F(0.055) will de- crease that at another frequency, but there were several exceptions. Three “hot spots”, D94, D196, and D274, found in [19] contributed to the amplitudes at frequen- cies F(0.295) and F(0.055) with different amounts (Ta b l e 3). There were a mutation T152V at the binding site, and two mutations S133T and S135A at the right edge of the their ∆A value was relatively small. Table 3 also con- tained several mutations of interest, which were T130K and S137P near the right edge of the binding pocket, and P183S, N184T, I185S, H193Q, T194N, and N196D near the active site. In [18], three networks of co-mutations in HA of 2009 H1N1 were uncovered. The first one had resi 276, and 309, the second one had residues 34, 167, 195, and 268, and the third one had residues 129, 210, and 238, where each residue co-mutated with others in the same network. Two pairs of mutations N167D/E195A and N269D/D276N in Table 2 were part of the afore- mentioned co-mutation networks discovered in [18]. Their individual and combined effects on ∆A were listed in Table 4, with the second pair having a much larger impact on the binding preference than the first. There were two clusters of mutations in Table 2, where the first was located at positions from 152 to 170, and the 00.05 0.10.15 0.20.25 0.30.350.40.450.5 0 1 2 3 4 5 6 IS of consensus HA1 sequence of 2009 H1N1 (pandemic human H1N1 binding) Frequency Amplitude 7 00.05 0.10.15 0.20.25 0.30.35 0.4 0.45 0.5 0 1 2 3 4 5 6 IS of consensus HA1 sequence of 2009 H1N1 (swine binding) Frequenc y Amplitude F(0.055) F(0.295  W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. 231 wine Binding DTICIGYHANNSTDTVDTVLEKNVTVTHSVNLLENSHNGKLCLLKGIAPLQLGNCSVAGW WLTGKNGLYPNLSKSYANNKEKEVLVLWGVH INYYWTLLEPGDTI c H1N1 (pandemic human H1N1 binding) and 2009 Table 3. Changes of amplitudes of IS frequencies by top 32 mutations with large ∆A value in HA1 of 2009 H1N1. Mutating Consensus HA1 Sequence of Swine Binding Patterns Mutating Consensus HA1 Sequence of Human Binding Patterns JBiSE second at positions from 257 to 278. The first cluster of mutations was contained in a pandemic human H1N1 receptor recognition domain (150:174) with the charac- teristic IS frequency at F(0.295) found in [22]. Prompted by this finding, we searched for a similar domain near the second cluster, and found a new domain (246:286) of swine binding characteristic with the IS dominant peak at frequency F(0.055) (Figure 3). S Human Binding DTLCIGYHANNSTDTVDTVLEKNVTVTHSVNLLEDKHNGKLCKLRGVAPLHLGKCNIAGW **:*******************************:.****** *:*:***:**:*.:*** Swine Binding ILGNPECELLISKESWSYIVEKPNPENGTCYPGHFADYEELREQLSSVSSFERFEIFPKE Human Binding ILGNPECESLSTASSWSYIVETSSSDNGTCYPGDFIDYEELREQLSSVSSFERFEIFPKT ******** * : .*******....:*******.* *********************** Right edge Swine Binding SSWPNH-TVTGVSASCSHNGESSFYRNLL Human Binding SSWPNHDSNKGVTAACPHAGAKSFYKNLIWLVKKGNSYPKLSKSYINDKGKEVLVLWGIH ****** : .**:*:*.* * .***:**:**. *.. **:***** *:* ********:* Left edge Swine Binding HPPNIVDQKTLYHTENAYVSVVSSHYSRKFTPEIAKRPKVRDQEGR Human Binding HPSTSADQQSLYQNADAYVFVGSSRYSKKFKPEIAIRPKVRDQEGRMNYYWTLVEPGDKI **.. .**::**:. :*** * **:**:**.**** **********:******:****.* Swine Binding IFEANGNLIAPRYAFALSRGFGSGIINSNAPMDKCDAKCQTPQGAINSSLPFQNVHPVTI Human Binding TFEATGNLVVPRYAFAMERNAGSGIIISDTPVHDCNTTCQTPKGAINTSLPFQNIHPITI ***.***:.******:.*. ***** *::*:..*::.****:****:******:**:** Swine Binding GECPKYVRSAKLRMVTGLRNIPSIQSR Human Binding GKCPKYVKSTKLRLATGLRNVPSIQSR *:*****:*:***:.*****:****** Figure 2.Alignment of two consensus HA1 sequences of 2009 pandemi seasonal H1N1 (swine binding). The binding sites in HA are colored in red, the left and right edges of the binding cleft in blue. Mutns atio∆A[F(0.055)]% ∆A[F(0.295)]% ∆A[F(0.055)]% ∆A[F(0.295)]% N35D 11.025 10.814 -12.123 -12.608 K45R -6.935 2.2999 8.1835 -6.5807 S56N -8.347 -9.5275 10.448 0.093211 L69S 1.1681 6.0652 -0.70212 -8.5586 I71S 7.0299 -9.42 - -7.5854 4.8054 E74S 8.719 - 3.4947-9.8488 9.1621 K82T 6.9799-4.8104 8.282 8. -5.4234 P83S -7.2536 7.4623 0. 6929-1.423 N84S -7.7848 880429.617 - 10.047 H94D 6.739 - 5.3039 7.836614.058 E120T 9.70842.2202 11.954 -6.9883 T130K -5.6997 7.272 5.909 - 5.7416 S137P 7.0562 0 8.60728.36474.9132 T152V .49754-6.8741 -3.6229 -9.9879 L157S -9.3159 -5.0672 10.114 -9.6759 P183S 6.6852 7.4566 0. -8.24462.4515 N184T 10.495 64815-11.043 -10.482 I185S H 9.2299 -10.327 -8.6728 3.045 3. 193Q -6.1179 4.6131 6.9435 7993 T194N 10.605 -4.89 -04 -9.784110.506 N196D -8.4752.04746.2191 16.521 N245T -5.3848 8.7494 9.7128 -10.596 L257M 9.7014 -7.2577 -10.573 10.05 4. S258E -8.2999-8.3169 8.7374 3745 N269D -9.1545 8.9086 6.7895 -12.176 M272V -3.1046 -9.3909 7.0087 9.0809 - D273H -6.1335 7.448 -10. 11.253 0.73203 K274D 7.7964 052-10.303 12.443 D276N -12.437-3.5437 13.913 9.3104 A277T 5.1287 - -7.8075 -4.46546.8946 R308K 0.592477.761 3.5286 -6.9255 M314L Total -7.2075 -27.0553 5.2935 0.9764 6.3119 40.4485 -5.2079 29.9315  232 W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. T Changeplitudes of IS frequby mutations containee co-mutation networ09 H1N1 Mutating Consensus HA1 Sequence of Swine Binding Patterns Mutating Consensus HA1 Sequence of Human Binding Patterns JBiSE able 4.s of amencies d in thks in 20 discovered in [18]. Mutations ∆A ∆A[]% [F(0.095)]%55)]% ∆A[F(0.2F(0.0.295)55)]% ∆A[F(0 N167D 0 0 0 0 E195A -2.674 -3.7307 2.1705 -0.36167 N1 A - N26 6N 67D, E1952. 0 -9.1545 674-37 8.9086 .730 2. 6.7895 1705 -0.7 -12.176 361 N269D D276N -12.437 -3.5437 13.913 9.3104 9D, D27-20.8003 4.3377 21.7617 -3.6715 00.05 0.10.15 0.20.25 0.3 0.35 0.40.450.5 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 Frequency Amplitude IS of a domain (246:286) of HA in 2009 with swine receptor interact Figure 3. IS of one domain (246:286) of swine binding . e set of HA1 sequences in avian H1N1 associated ion characteristic in HA1 of 2009 H1N1. 1.2. Avian H5N1 3 Although the whol H5N1 (n=1228) displayed the CIS dominant peak at frequency F(0.076) (Figure 4(a)), there were several 00.05 0.10.15 0.2 0.25 0.3 0.35 0.4 0.45 0.5 0 50 100 150 200 250 300 CIS of HA1 of avian H5N1 (avian binding) F(0.055 F(0.076) S/ N Frequency (c) 00.05 0.1 0.15 0.20.25 0.3 0.35 0.4 0.45 0. 5 0 1 2 3 4 5 6 Frequency Ampl itude IS of consensus HA1 sequence of avian H5N1 (seasonal human H1N1 binding) F( (a) (b) (d) 00.05 0.10.15 0.2 0.25 0.30.35 0.4 0.45 0.5 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 Frequency Amplitude IS of consensus HA1 sequence of avian H5N1 (avian binding) (e) Figure 4. (a) CIS of consensus of all HA1 sequences of avian H5N1. (b) CIS of consensus HA1 sequence of avian H5N1 with seasonal human H1N1 binding. (c) CIS of consensus HA1 sequence of avian H5N1 with avian binding. (d) IS of consensus HA1 sequence of avian H5N1 with seasonal human H1N1 binding. (e) IS of consensus HA1 sequence of avian H5N1 with avian binding. CIS of HA1 of avian H5N1 (all sequences) 00.05 0.10.15 0.20.25 0.3 0.35 0.4 0.45 0.5 0 50 100 150 200 250 300 Frequency S/N F(0.076) 00.05 0.10.15 0.20.25 0.30.35 0.4 0.45 0.5 0 50 100 150 200 250 300 CIS of HA1 of avian H5asonal human H1N1 binding) Frequency S/ N N1 (se 0.236) F(0.076) F(0.236)  W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. 233 HA1ak at frcy utation between avian and human binding patterns in of Avian Binding Patterns of Human Binding Patterns JBiSE sequences in the dataset that had a higher IS pe equency F(0.236) than that at the frequen F(0.076). Bases on this observation, the whole set of HA1 sequences in avian H5N1 collected were divided into two subsets. One had the IS dominant peak at fre- quency F(0.076), referred to as avian binding subset (n=949), and the other had the IS dominant peak at frequency F(0.236), referred to as human binding sub- set (n=279). The CIS of these two subsets of HA1 se- quences were plotted in (b) and (c) of Figure 4, and the IS of their consensus HA1 sequences were plotted in (d) and (e) of Figure 4, respectively. A total of 11 amino acid changes between the two consensus HA1 sequences of avian binding and human binding were found, and the re- sulting amplitude variation from each mutation was com- puted (Table 5). There were several mutations near the active site: D154N, N155S, A156T, and R189K. Table 5. Changes of amplitudes of IS frequencies by each m HA1 of avian H5N1. 3.1.3. Avian H1N1 For avian H1N1, we used the same strategy as for avian H5N1 to divide the whole set of HA1 sequences (n=78) in this subtype into two subsets according to different binding patterns (human (n=19) vs. avian (n=59)) as reflected by two different IS characteristic frequencies (F(0.295) vs. F(0.282))(Figure 5). Between the two con- sensus HA1 sequences of avian (F(0.282)) and human (F(0.295)) binding, there was only one mutation N121S (Table 6). The ISM was applied to quantify this muta- tion’s contribution to the amplitude variation of the consensus HA1 sequence in each binding characteristics (Table 6). For H1N1 viruses, the substitutions E190D/G225D were essential for avian virus HA to acquire human virus receptor specificity [13]. Here ISM was employed to verify this fact numerically (Table 7). Mutating Consensus HA1 Sequence Mutating Consensus HA1 Sequence Mutations ∆A[F(0.076)]% ∆A[F(0.236)]% ∆A[F(0.076)]% ∆A[F(0.236)]% L 71I 0 00 0 I83A -2.7308 3.8849 2.6972 -3.2926 R140K -7.3 5.8.-45 - - 118 -8.7 -109 7. 8142052 2224 .356 D154N -14.371 5.9825 15.75 -3.3428 N155S 6.3122 9.7463 -5.5234 -8.0289 A156T 1.9481 4.9408 1.3726 -3.7429 R189K 0.21422 -7.9896 0.36709 7.2805 N252Y -5.9842 3.5318 6.173 -3.0255 T262A 0 0 0 0 I282M 4.7565 10.576 4.6209 -8.6614 G323R .34071.85048 Total -6.3212 27.8086 10.8338 -20.1221 Table es of amplitudes tation between an bin HA1 of avian H1N1. of Avian Binding Patterns of Human Binding Patterns 6. Changof IS frequencies by each muavian and humding patterns in Mutating Consensus HA1 Sequence Mutating Consensus HA1 Sequence Mutation ∆A [ ∆A [)]% F(0.282)]% ∆A [F(0.295)]%F(0.282)]% ∆A [F(0.295 N121S -15.71 18.6142 18.625439 -15.7008 Tab anges of s of IS frequenutations E190D/ Mutating Consensus HA1 Sequences of Avian H1N1 le 7. Ch amplitudecies by mG225D. Mutations ∆A [F(0.282)]% ∆A [F(0.295)]% Dominant Peak Frequency E190D -13.2024 F(0.282) 13.0862 G225D -10.4239 -0.6548 F(0.282) E19D 0D,G225-22.8997 11.7994 F(0.295) 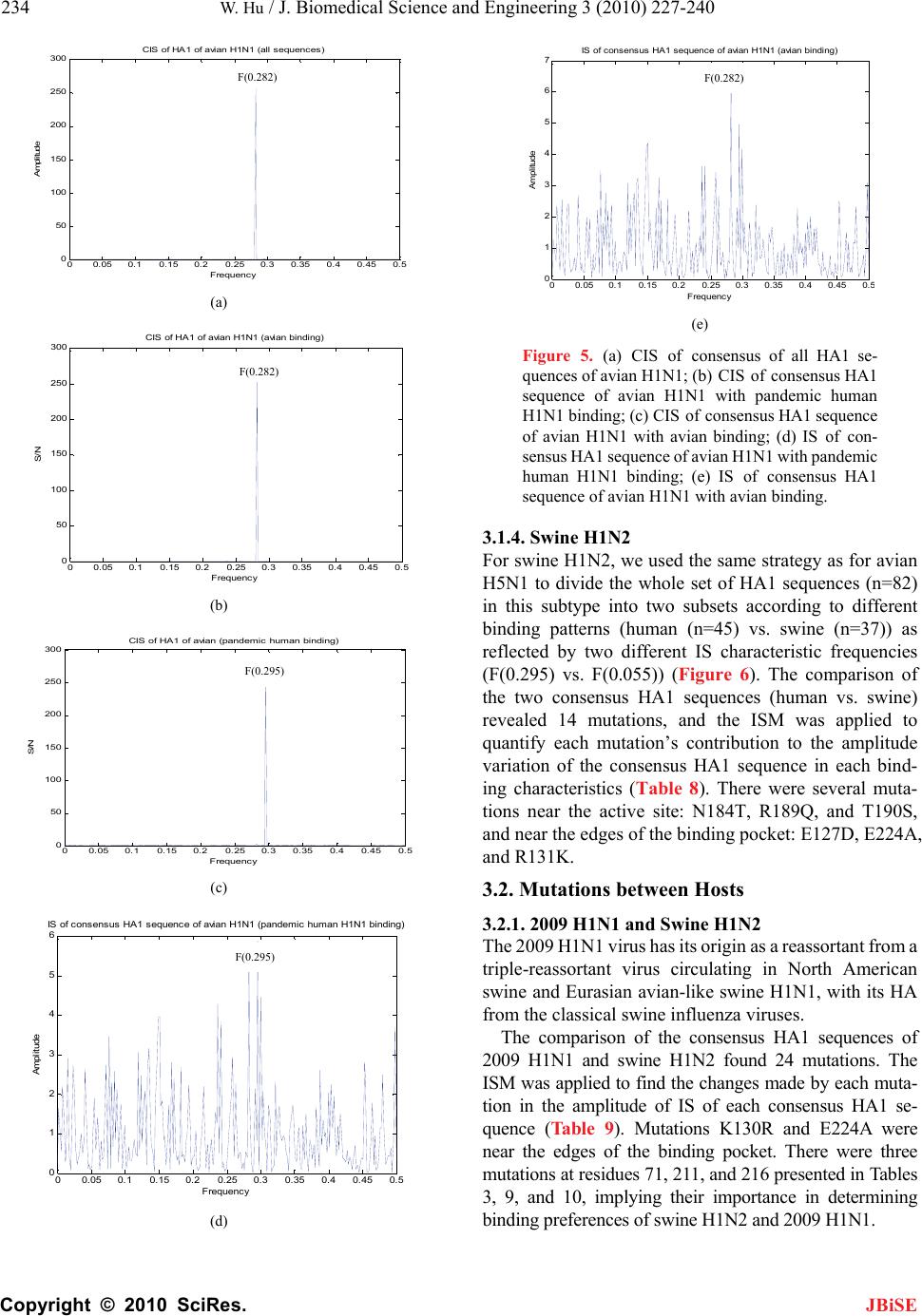 234 W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. JBiSE (a) (b) 00.05 0.10.15 0.20.25 0.30.35 0.4 0.45 0.5 0 50 100 150 200 250 300 S/N Frequency (c) CIS of HA1 of avian (pandemic human binding) 00.05 0.10.15 0.20.25 0.30.350.40.450.5 0 1 2 3 4 5 6 Frequency Amplitude IS of consensus HA1 sequence of avian H1N1 (pandemic human H1N1 binding) (d) 00.05 0.10.15 0.20.25 0.30.35 0.4 0.45 0. 0 1 2 3 4 5 6 7 IS of consensus HA1 sequence vian binding) Frequency Ampl i tude (e) Figure 5. (a) CIS of consensus of all HA1 se- quences of avian H1N1; (b) CIS of consensus HA1 sequence of avian H1ith pandemic human H1N1 binding; (c) CIS of consensus HA1 sequence of avian H1N1 with avian IS of con- sensus HA1 sequence of avian H1N1 with pandemic human H1N1 binding; (e) IS of consensus HA1 sequence of avian H1N1 with avian binding. 3.1.4. Swine H1N2 For swine H1N2, we used the same strategy as for avian H5N1 to divide the whole set of HA1 sequences (n=82) in this subtype into two subsets according to different binding patterns (human (n=45) vs. swine (n=37)) as reflected by two different IS characteristic frequencies (F(0.295) vs. F(0.055)) (Figure 6). The comparisn of e two consensus HA1 sequences (human vs. swine) eved to quan tude varia nd- ing cuta- tions90S, and n4A, and R tween Hosts of avian H1N1 (a N1 w binding; (d) o th raled 14 mutations, and the ISM was applie tify each mutation’s contribution to the ampli tion of the consensus HA1 sequence in each bi haracteristics (Table 8). There were several m near the active site: N184T, R189Q, and T1 ear the edges of the binding pocket: E127D, E22 131K. 3.2. Mutations be 3.2.1. 2009 H1N1 and Swine H1N2 The 2009 H1N1 virus has its origin as a reassortant from a triple-reassortant virus circulating in North American swine and Eurasian avian-like swine H1N1, with its HA from the classical swine influenza viruses. The comparison of the consensus HA1 sequences of 2009 H1N1 and swine H1N2 found 24 mutations. The ISM was applied to find the changes made by each muta- tion in the amplitude of IS of each consensus HA1 se- quence (Table 9). Mutations K130R and E224A were near the edges of the binding pocket. There were three mutations at residues 71, 211, and 216 presented in Tables 3, 9, and 10, implying their importance in determining binding preferences of swine H1N2 and 2009 H1N1. CIS of H 300 00.05 0.10.15 0.20.25 0.30.35 0.4 0.45 0.5 0 50 100 150 200 250 A1 of avian H1N1 ( Frequency Amplitude all sequences) F(0.282) F(0.282) 300 00.05 0.1 0.15 0.20.25 0.3 0.350.40.450. 0 50 100 150 200 250 Frequenc y S/ N CIS of HA1 of avian H1N1 (avian binding) F(0.282) F(0.295) F(0.295)  W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. 235 JBiSE 00.05 0.10.15 0.20.25 0.30.35 0.4 0.45 0.5 0 20 40 60 80 100 120 140 Frequency (a) (a) S/N CIS of HA1 of swine H1N2 (all sequences)) 00.05 0.10.15 0.20.25 0.3 0.35 0.4 0.45 0.5 0 50 100 150 200 250 300 Frequency S/N (b) CIS of HA1 of swine H1N2 (swine binding) 00.05 0.10.15 0.20.25 0.3 0.350.4 0.45 0.5 0 50 100 150 200 250 300 Frequenc y S/N CIS of HA1 of swine H1N2 (pandemic human H1N1 binding) (c) 00.05 0.10.15 0.20.25 0.30.350.40.450.5 0 1 2 3 4 5 6 7 IS of consensus HA1 sequence of swine H 00.05 0.10.15 0.20.25 0.30.350.40.450.5 0 1 2 3 4 5 6 7 Frequenc y Amplitude IS of consensus HA1 sequence of swine H1N2 (swine binding) 1N2 (pandemic human H1N1 binding Frequency Amplitude (d) (e) Figure 6. (a) CIS of conssus of all HA1 sequences of swine H1N2; (b) CIS of consensus HA1 sequence of swine H1N2 with pandemn H1N1 binding; (c) CIS of consensus HA1 se swine H1N2 with swine binding; (d) IS of consensus HA1 sequence of swine H1N2 with pandemic human H1N1 binding; (e) IS of consensus HA1 sequence of swine H1N2 with swine binding. en ic huma quence of 00.05 0.10.15 0.20.25 0.3 0.35 0.4 0.45 0.5 0 1 2 3 4 5 6 IS of consensus HA1 sequence of 2009 H1N1 Frequency Ampl i t ude (a) 00.05 0.10.15 0.20.25 0.3 0.35 0.4 0.45 0.5 0 1 2 3 4 5 6 7 IS of consensus HA1 sequence of swine H1N2 ude Figu consensus HA1 sequence of 2009 H1N1. (b) IS of consensus HA1 sequence of swine H1N2. Mutations in the binding site not only could modulate receptor specificity but also the antigenicity of the virus [27]. It was generally believed that the key residues at the binding site were not subject to selection, but re- cently a strong positive selection at position 190 in HA of H1N1 was detected in [13]. It was observed that there Frequenc y Amplit (b) re 7. (a) IS of F(0.055) F(0.295) F(0.055) F(0.055) F(0.295) F(0.295) F(0.295) F(0.055) F 0.295  236 W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. Table 8. Changes of amplitudes of IS frequencies by each mutation between swine and human binding patterns in HA1 of swine H1N2. Mutating Consensus HA1 Sequence of Swine Binding Patterns Mutating Consensus HA1 Sequence of Human Binding Patterns Mutations ∆A [F(0.055)]% ∆A [F(0.295)]% ∆A [F(0.055)]% ∆A [F(0.295)]% V47I -0.45253 -0.39918 0.61877 0.4217 I71F -7.4498 3.7605 9.5403 -3.7394 E127D -11.057 -1.2554 14.693 3.0055 R131K 0 0 0 0 G170E 0.014238 -0.07755 -0.01469 0.07289 N184T -8.3652 -10.1 R189Q -1.4448 -2.6783 T190S -1.1476 0.1999 T211K -6.1626 6.441 K216T -1.9443 7.4225 E224A -0.10535 -1.9348 A250V -1.1944 -3.5238 P271S -2.8719 3.2365 K278T -0.95454 0.60359 Total -43.1358 1.6950 11.495 10.475 1.6451 2.6875 1.352 -0.09641 8.1349 -6.0602 2.3502 -6.895 0.42226 1.7602 1.8901 3.4949 3.4537 -3.3317 2.0448 -0.81079 57.6254 0.9842 were two distinct evolutionary patterns in host-driven antigenic drift of human H1N1 HAs at positions 190 and 225, i.e., the antigenic drift of 1918 pandemic HAs oc- curred at position 225, and that of epidemic HAs hap- pened at position 190. In contrast to these two trends, the HAs in 2009 H1N1 took a different path, which were highly conserved at both positions 190 and 225, based on the 73 HA sequences of 2009 H1N1, as of July 10, Mutating Consens Mutating Consens09 H1N1 Table 9. Changes of amplitudes of IS frequencies by each mutation between consensus HA1 sequences of 2009 H1N1 and swine H1N2. us HA1 Sequence of Swine H1N2us HA1 Sequence of 20 Mutations ∆A[F(0.055)](0.295)]% ∆A[F(0.0[F(0.295)]% % ∆A[F55)]% ∆A K36R -5.6796 5.4297 7.241-4.7622 2 I61L 0 0 0 0 S71F -0.2 S84N -7 D97N -4 T-8 2 K130R -4 K142N 0.8 K146R -5 D168N 4.7 G0.0- 8 R205H 1. 3 K6 I216K -1 E224A -08 K2-5 4 M257L 8.8688 -8.1637 -10.571 10.056 -0.05099 0.1242 0.071321 -0.12802 A261S 1.0216 0.76162 -0.593 -1.5978 I298V 0.093799 0.2947 -0.08087 -0.42787 K302E -3.1322 1.8998 4.0108 -1.4074 L314M -4.0997 6.3337 6.3078 -5.2087 V321I -0.49821 -0.48678 0.6555 0.49537 Total -31.7389 -27.3764 45.9990 28.7656 75109 .0341 0.59143 -8.3814 0.9072 9.614 -0.85027 10.052 .8444 -9.6342 6.04519.9181 120E .911 5.4563 11.964-7.003 .3521 -4.4943 6.0754 5.9088 67563 -2.0369-0.36042.8758 .7743 -2.0864 7.40971.4689 5193 -5.5381 -5.7444.824 170E 11884 0.07133-0.01050.06883 7024 . 1.1275 -2.4361 4 -2.393 211T 5905 -5.7011 -7.8046.4193 .8394 3.6983 2.0037-3.8566 .2376 -1.7644 0.61941.4082 39T .1394 0.52104 6.89240.8922 E258K -2.8787 -2.2561 3.7825 2.0134 N260G JBiSE  W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. 237 2009. In the pesent stu had the following coun tions 190in HA 1 (Table appeared t09 H1Ncontinued to ke this evoluti pattern. The alteration of receptor pecificity was lieved to bssential step adaptation. Seve studies on HA discove a single mutat D225G de the bindingty of 1918 HA α2,6 recept resulted in α2,6/α2,3 bindi virus, furte, a double190E/D22 abolished ing of 1918 α2,6 and resulted a α2,3 binirus [28,29umerical anal (Table 11)ted that the ns at positions 1 and 225 woduce similang affinity of 20 H1N1 HAf 1918 tation D190E Table 10. Mutation counts in HA1 sequences of 2009 H1N Mutation Counts JBiSE rdy, as of November 20, 2009, we ts of various mutations at posi- and 225 of 2009 H1N10). It hat the 201 HAs ep onary binding sbe- e an ein host ral 1918 red thation creased affinifor ors and a mixedng hermor mutations D5G the bindHA to in ding v]. Our nysis suggesmutatio90 ould prr bindi09 to that oHA. Mude- 1. D190G 1 D190V 5 D226 D225G 7 D225N 2 5E Table 11. Changes of amplitudes of IS frequencies by muta- tions observed in HA1 sequences of 2009 H1N1. Mutating Consensus HA1 Sequence of 2009 H1N1 Mutations ∆A [F(0.055)]%∆A [F(0.295)]% Peak Frequency Dominant D190E -2.0061 -9.6206 F(0.282) D190G -2.0302 -9.6888 F(0.282) D190N -2.0789 -9.8255 F(0.282) D190V -2.0027 -9.6109 F(0.282) D225E -9.5672 -2.1233 F(0.295) D225G -9.6345 -2.1488 F(0.295) D225N -9.7694 -2.2003 F(0.295) D190E,D225E -10.5225 -12.3705 F(0.282) D190E,D225G -10.5836 -12.3981 F(0.282) D190E,D225N -10.7061 -12.4537 F(0.282) D190G,D225E -10.5392 -12.4420 F(0.282) D190G,D225G -10.6002 -12.4696 F(0.282) D190G,D225N -10.7226 -12.5253 F(0.282) D190N,D225E -10.5729 -12.5854 F(0.282) D190N,D225G -10.6338 -12.6131 F(0.282) D190N,D225N -10.7560 -12.6688 F(0.282) D190V,D225E -10.5201 -12.3603 F(0.282) D190V,D225N -10.7037 -12.4435 F(0.282) D190V,D225G -10.5812 -12.3879 F(0.282) crea that at f sedre than binding finity, whD225G opposite effect, exhibituman bindiificity. The double mutations190E/D225G show avian binding preference. 3.2.2. Avian Hd Human H5N Most of the hithogenic avianrains bind strongly to aviators. This specificity is normally a barrier to virmission fromto humans. However, a few have been di to bind to human receptoll as to avian rrs. The com- parison of thes HA1 sequean H5N1 and human Hentified three ns, R140K, R189K, and T2nd their impacharac- teristic frequencies F(0.076) and F(0.s illustrated in Table 12. As demonstthe experimenucted in ref- erences [5,6], utions Q192R223L could mediate a shiian to humanpreference in the H5N1 vin references [3], mutations Q226L and Ganced the huding capac- ity while redu binding capf the H5N1 viruses. Here t was utilized ate the out- come of these ns (Table 13). illustrated the effect of m S223L on the twoaracteristic frequencies F(0.076) and F(0.236). 4. DISCUSSION Under the minimal adaptivehanges necessary for virtation to human host isf key importance in learw pandemic influenza uses emerge. Al- teratioceptor recognition is ital step in host daptahich is modulated directly by a subset of nd op- timize tissue tropism There are severans in HA pro- tein in different flu subtypes, playing critical roles in rec inhu HA, thsylatio HA m e bindificity of [32]. Ttationsv- ered in thy represenalternativ which can switch its subgnitioese ms occur i, wheose artificially engineere wet niquesve a v probo occur in nature. Asced bydy in [30] that muta226L a8S in Hns cr thecifithe H5ses. - leinted the li to ae neotids to pese s ins is sich con iny the amplitude at frequency F(0.295) mo requency F(0.055), displaying the avian afile mutation produced the ing the hng spec Ded 5N1 an1 ghly pa H5N1 st n recep al trans birds of themscovered rs as weecepto consensunces of avi 5N1 idmutatio 63A, at on the two c 236) wa rated in ts cond substit and S ft from av binding ruses. I0,31,32 228S enhman bin ced avianacity o he ISMto evalu mutatio Figure 8 utation ch standing c al adap o ning hovir n of re tion, w a v a amino acids present in the HA protein. Other determinants of host adaptation include continued viral evolution to improve transmission and replication efficiency a . l well-known mutatio eptor bind e glyco g preference s n sites in ift. Besides m ight also tations in impact th ng speci HAhe mu disco is studt es by the HA strate recon. Thutation n nature lab tech reas th may ha d by ability tery low eviden the stu tions Qnd G22A proteiould alte e sp ss, it also po bindingcity of N1 viruNeverthe out thatkelihoodcquire th ecessary nucle changeroduce thmutation natural virumall, whuld explai part wh  238 W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. hang litudesuench muta 1. ing C 1 Seq vian JBiSE Table 12. Ces of amp of IS freqies by eaction between consensus HA1 sequences of avian H5N1 and Mutating Consensus HA1 Sequence of Human H5N1 human H5N Mutatonsensus HAuence of A H5N1 Mutation ∆A [F((0.236)]% ∆A [F(0.236)]% 0.076)]% ∆A [F% ∆A [F(0.076)] R140K 7.94.5900 5.2052 425 --7.8143 R189K -0.7370 -3.6.0611 41 5253 7.0.2142 -7.9896 4.5619 6.8405 -3.0382 4.0561 T263A7894 - Total 3.6278 -2.91 Table 13. Changes of amplitudes of IS frequencies by muta- tions experimented in references [5,6,30,31,32]. Mutating Consensus HA1 Sequence of Avian H5N1 Mutations ∆A [F(0.076)]% ∆A [F(0.236)]% Q192R -3.2185 1.5596 S223L -4.0399 10.8075 Q192R, S223L -6.5711 11.6823 Q226L 0.9498 17.4463 G228S 7.3516 15.7656 Q226L, G228S 8.0339 16.9010 (a) 00.05 0.10.15 0.20.25 0.30.35 0.40.45 0.5 0 0. 5 1 1.5 2 2.5 3 3.5 4 4.5 5 Frequency Amplitude of consensus HA1 sean H5N1 an human transmission, anolecular determi- nants are required. Inosed that binding to long-chain α2-6 sialessary requirement viruses to efficienlicate and hu- ans. Thery in asseption to human receptoty from the analysis of a few in- fluenza strainsIt showed that ects of the same mutation as Q226L/G228the binding preference of in were not th on others strains in H5N1. Because lab experiments are labor in- tenly, bioincs approachrea- sonable alternatives in the af binding specificity given the large number of virus sequences, which can process all strains with any combination of mutations within a subtype efficiently. 5. CONCLUSIONS The increasing trend of direct transmission of avian/ swine influenza viruses to humans underscores the need to understand further the mechanism of glycan receptor recognition and specificity switch. In this study, muta- IS quence of hum (b) (c) Figure 8. (a) IS of consensus HA1 sequence of avian H5N1; (b) IS of consensus HA1 sequence of human H5N1; (c) IS of consensus HA1 sequence of avian H5N1 mutated by S223L. viruses such as H5N1 have not yet evolved into human ransmissible strains to cause a human pandemic. t It appears that increased binding affinity for hum influenza receptors alone is not sufficient for efficient d additional m [3op3], it was pr osides is a nec for m tly rep e is inadequac transmit in ssing the ada r affini [30]. the eff s, suchS, on one strae same sive and costformaties offer nalysis o 00.05 0.10.15 0.20.25 0.3 0.35 0.4 0.45 0.5 0 1 2 3 4 5 6 IS of mutated consensus HA1 sequence of avian H5N1 (S223L) Frequency Amplitude F(0.076) F(0.236) 0.4 0.45 0.500.05 0.10.15 0.20.250.3 0.35 0 1 2 3 4 5 6 IS of consensus HA1 sequence of avian H5N1 Frequency Amplitude F(0.076) F(0.236) F(0.076) F(0.236)  W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. 239 tions in HA1 within or between hosts in various flu sub- types were identified, and their contribution to binding preference asuredthe ISM. Our numerical analysis implied that the mutations in HA1 of 2009 hu- man H1N1 collectively tended to reduce the swine binding affinity in the seasonal H1N1 strains and to in- crease that in the pandemic H1N1 strains. At the same time, they increased the human binding affinity in the pandemic H1N1 strains and had little impact on that in the seasonal H1N1 strains. The mutations in HA1 of avian H5N1 and avian H1N1 exhibited reduced avian binding in the strains of avian binding propensity while showed enhanced avian binding affinity in the strains with human binding propensity. They displayed thep- posite effects on human big. The mutations in HA1 of swine H1N2 reduced theine binding affinity in the strains with swine binding propensity and enhanced that in the strai human binding propensity. They showed little pact on tman binding affinity, which was different from the mutations in HA1 of 2009 H1N1. The mutationsbetween the consensus HA1 se- quences of 2009 H1N1 and swine H1N2 decreased both the human and swine binding affinities in swine H1N2 and increased those in 2009 H1N1. The mutations be- tween the consensus HA1 sequences of human H5N1 and avian H5N1 increased the avian binding affinity and decreased the human binding affinity in avian H5N1 while produced the opposite effects on those in human H5N1. The mutations discovern the present study c firmed the potential for influenza viruses to adapt to human host, and furthermore, our numerical analysis detailed the extent of binding preference changes in- duced by eutation. mutations and their cor- responding contribution to the binding specificity altera- tion yielded new clues to the mechanism of receptor recognition switch within and between hosts. The ISM offered a complementary and efficient approach to in- vestigate the binding affinities of all HA sequences in various subtypes, a task difficult to accomplish experi- mentally. 6. ACKNOWLEDGMENTS We thank Houghton College foinancial support. RE [1] gs of the National Academy of Sciences, USA, 96(4), 1164-1166. ovin, N., Gambaryan, A., Early alterations of the re- es of H1, H2, and H3 avian in- 09) Extrapolating from sequence–the 2009 H1N1 was me using JBiSE o ndin sw ns with imhe hu ed ion- ach mThese r its f FERENCES Webster, R.G. (1999) 1918 Spanish influenza: The secrets remain elusive. Proceedin [2] Li, Q., Kash, J.C., Dugan, V.G., Wang, R.X., Jin, G.Z., Cunningham, R.E. and Taubenberger, J.K. (2009) Role of sialic acid binding specificity of the 1918 influenza virus hemagglutinin protein in virulence and pathogene- sis for mice. Journal of Virology, 83(8), 3754‐3761. [3] Matrosovich, M.N., Klenk, H. D. and Kawaoka, Y. (2006) Receptor specificity, host-range, and pathogenicity of in- fluenza viruses. In Yoshihiro Kawaoka (ed.) Influenza Virology: Current Topics, Caister Academic Press, 95‐137. [4] Zambon, M. (2007). Lessons from the 1918 influenza. Nature Biotech, 25, 433‐434. [5] Gambaryan, A. Tuzikov, A., Pazynina, G., Bovin, N., Balish, A. and Klimov, A. (2006) Evolution of the re- ceptor binding phenotype of influenza A (H5) viruses. Virology, 344(2), 432‐438. [6] Yamada, S., Suzuki, Y., Suzuki, T., Le, M. Q., Nidom, C. A., Sakai-Tagawa, Y., Muramoto, Y., et al. (2006) Hae- magglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Na- ture, 444(7117), 378‐382. [7] Matrosovich, M., Tuzikov, A., B and Klimov, A., et al. (2000) ceptor-binding properti fluenza virus hemagglutinins after their introduction into mammals. Journal of Virology, 74(18), 8502‐8512. [8] Bateman, A.C., Busch, M.G., Karasin, A.I., Bovin, N., and Olsen, C.W. (2008) Amino acid 226 in the hemag- glutinin of H4N6 influenza virus determines binding af- finity for alpha2, 6-linked sialic acid and infectivity lev- els in primary swine and human respiratory epithelial cells. Journal of Virology, 82(16), 8204‐8209. [9] Wan, H., Sorrell, E.M., Song, H., Hossain, M.J., Rami- rez-Nieto, G., et al. (2008) Replication and transmission of H9N2 influenza viruses in ferrets: Evaluation of pan- demic potential. PLoS ONE, 3(8), e2923. [10] Rogers, G.N. and D’Souza, B.L. (1989) Receptor binding properties of human and animal H1 influenza virus iso- lates. Virology, 173, 317‐322. [11] Matrosovich, M.N., Gambaryan, A.S., Teneberg, S., Piskarev, V.E., Yamnikova, S.S., et al. (1997) Avian in- fluenza A viruses differ from human viruses by recogni- tion of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Vi- rology, 233(1), 224‐234. [12] Reid, A.H., Janczewski, T.A., Lourens, R.M., Elliot, A.J., Daniels, R.S., Berry, C.L., Oxford, J.S. and Taubenber- ger, J.K. (2003) 1918 influenza pandemic caused by highly conserved viruses with two receptor-binding variants. Emerg Infect Disease, 9(10), 1249‐1253. [13] Shen, J., Ma, J. and Wang, Q. (2009) Evolutionary trends of A(H1N1) influenza virus hemagglutinin since 1918. PLoS ONE, 4(11), e7789. [14] Srinivasan, A., Viswanathan, K., Raman, R., Chandra- sekaran, A., Raguram, S., Tumpey, T.M., Sasisekharan, V. and Sasisekharan, R. (2008) Quantitative biochemical ra- tionale for differences in transmissibility of 1918 pan- demic influenza A viruses. Proceedings of the National Academy of Sciences, 105(8), 2800-2805. [15] Soundararajan, V., Tharakaraman, K., Raman, R., Ragu- ram, S., Shriver, Z., Sasisekharan, V. and Sasisekharan R. (20 “swine” influenza virus. Nature Biotechnology, 27(6), 510‐513. [16] Childs, R.A., Palma, A.S., Wharton, S., Matrosovich, T., Liu, Y., Chai, W.G., Campanero-Rhodes, M.A., Zhang, Y.B., Eickmann, M., Kiso, M., Hay, A., Matrosovich. M. and Feizi, T. (2009) Receptor-binding specificity of  240 W. Hu / J. Biomedical Science and Engineering 3 (2010) 227-240 Copyright © 2010 SciRes. al Science and Engineering, 2(7), 550‐558. een the 2009 H1N1 aminidase: Mutations ournal of n, H.L., Glisic, S., Veljkovic, N., Per- er C.P. (2009). Identification of hemag- nza viruses: 2009 H1N1, ment in accuracy of mu ay-Nedecký, G., Karol Haver- alyses of influenza virus hae- eedings of the eals different receptor specificities. Journal of (2006) Glycan microarray analysis of the JBiSE pandemic influenza A (H1N1) 2009 virus determined by carbohydrate microarray. Nature Biotechnology, 27 (9), 797‐799. [17] Hu, W. (2009) Analysis of correlated mutations, stalk motifs, and phylogenetic relationship of the 2009 influ- enza A virus neuraminidase sequences. Journal of Bio- medic [18] Hu, W. (2010) The Interaction betw influenza A hemagglutinin and neur, Bico-mutations, and the NA stalk motifs. J medical Science and Engineering, 3, 1‐12. o- magglutinins with different receptorbinding specificities. Virology, 138, 174‐177. [28] Srinivasan, A., Viswanathan, K., Raman, R., Chandrase- karan, A., Raguram, S., et al. (2008) Quantitative bio- chemical rationale for differences in transmissibility of 1918 pandemic influenza A viruses. Proc [19] Veljkovic, V., Nima ovic, V. and Mull glutinin structural domain and polymorphisms which may modulate swine H1N1 interactions with human re- ceptor. BMC Structural Biology, 9, 62. [20] Veljkovic, V., Veljkovic, N., Muller, C.P., Müller, S. Glisic, S., Perovic, V. and Köhler, H. (2009) Characteri- zation of conserved properties of hemagglutinin of H5N1 and human influenza viruses: Possible consequences for therapy and infection control. BMC Structural Biology, 7, 9‐21. [21] Cosic, I. (1997) The resonant recognition model of mac- romolecular bioreactivity, theory and application. Berlin: Birkhauser Verlag. Hu, W. (2010) Identification of highly conserved do-[22] mains in hemagglutinin associated with the receptor binding specificity of influe avian H5N1, and swine. Journal of Biomedical Science and Engineering,3, 114-123. [23] Katoh, K., Kuma, K., Toh, H. and Miyata, T. (2005) MAFFT version 5: Improveltiple [32] aulson, J.C., et al. (2008) Recent avian H5N1 viruses exhibit in- creased propensity for acquiring human receptor specific- ity. Journal of Molecular Biology, 381(5), 1382‐1394. sequence alignment. Nucleic Acids Research, 33(2), 511‐ 518. [24] MacKay, D. (2003) Information theory, inference, and learning algorithms. Cambridge University Press. [25] KováccaronOVá, A., Ruttk líK, I. and Janecccaronek, S. (2002) Sequence similari- ties and evolutionary relationships of influenza virus A hemagglutinins, Virus Genes, 24(1), 57‐63. [26] Gamblin, S.J., Haire, L.F., Russell, R.J., Stevens, D.J., Xiao, B., Ha, Y., et al. (2004) The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science, 303, 1838‐1842. [27] Daniels, R.S., Douglas, A.R., Skehel, J.J., Wiley, D.C., Naeve, C.W., Webster, R.G., Rogers, G.N. and Paulson, J. C. (1984). Antigenic an National Academy of Sciences, 105, 2800‐2805. [29] Stevens, J., Blixt, O., Glaser, L., Taubenberger, J.K. , Pal- ese, P., et al. (2006) Glycan microarray analysis of the hemagglutinins from modern and pandemic influenza viruses rev Molecular Biology, 355(5), 1143‐1155. [30] Ayora-Talavera, G., Shelton, H., Scull, M.A., Ren, J., Jones, I.M., et al. (2009) Mutations in H5N1 influenza virus hemagglutinin that confer binding to human tra- cheal airway epithelium. PLoS ONE, 4(11), e7836. [31] Stevens, J., Blixt, O., Glaser, L., Taubenberger, J.K., Pal- ese, P., et al. hemagglutinins from modern and pandemic influenza viruses reveals different receptor specificities. Journal of Molecular Biology, 355(5), 1143‐1155. Stevens, J., Blixt, O., Chen, L.M., Donis, R.O., P [33] Chandrasekaran, A., Srinivasan, A., Raman, R., Viswa- nathan, K., Raguram, S., Tumpey, T.M., Sasisekharan, V. and Sasisekharan, R. (1008) Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nature Biotechnology, 26(1), 107‐113.
|