Paper Menu >>
Journal Menu >>
 J-S Paquette et al. Human asthmatic bronchial equivalents Abstract The isolation of human bronchial epithelial (HBEC) and fibroblastic cells (HBFC) from biopsies of asthmatic and non-asthmatic volunteers provided unique cellular materi- als to be used for the production of bioengineered bron- chial equivalents (BE) in vitro. The HBEC are grown on a mesenchymal layer seeded with HBFC and the BE can be maintained for at least 15 days in culture. Under the BE culture conditions established previously, HBEC undergo differentiation into ciliated and goblet cells, within a pseudostratified organization comparable to human bron- chi. We published previously the results from histologic and functional analyses of such BE produced exclusively using non-asthmatic HBEC and HBFC. We report here the com- parative analyses of BE produced with non-asthmatic and asthmatic living HBEC and HBFC (naBE and aBE, respec- tively). Our data indicated that all asthmatic HBEC populations grown on a mesenchymal layer, containing non- asthmatic HBFC, slowly reached a confluent state but then detached from the matrix upon culture time. These BE ap- pear to be very good models to study the mechanisms in- volved in asthma in vitro. Key words: human bronchial equivalents, asthma, bioen- gineering. Introduction In our modern society, thousands of people suffer from mild to severe asthma. This bronchial disorder is mostly associated with mucosal inflammation and airway hyperresponsiveness (Jeffery et al., 1989; Djukanovic et al., 1990; Boulet et al., 1993). Histologic analyses of bron- chial biopsies taken on asthmatic patients report an appar- ent basement membrane thickening most likely caused by sub-epithelial fibrosis. It is postulated that fibroblasts, in- volved in collagen synthesis and remodelling, could be responsible for the acute fibrosis in response to cytokines secreted by inflammatory or epithelial cells (Brewster et al., 1990; Roche, 1991; Gauldie et al., 1992). Another major change in the structural properties of asthmatic bron- chi is their partial or complete desquamation upon the evo- lution of the disease (Jeffery et al., 1989). Up to now, the alterations of the bronchial tissues of asthmatic subjects remain poorly understood and the various hypotheses raised on the putative mechanisms responsible for the mainte- nance and the progression of these changes have to be as- sessed. Besides live animals (often rats and dogs) (DiCosmo et al., 1995; Chung, 1996; Widdicombe, 1996; Shichinoke et al., 1996), several research groups use animal bronchial tissues (Opazo-Saez and Pare, 1994; Baeza-Squiban et al., 1994; Davenport and Nettesheim, 1996) or cells grown in monolayers (De Jong et al., 1994; Gray et al., 1996) as experimental models, to study various aspects of asthma in vitro. To overcome interindividual variations among animals used as experimental models, the number of sub- jects needed to perform each study has to be quite consid- erable. Moreover, animal models are complex and it be- comes sometimes difficult to control all physiologic pa- rameters which may modulate the results of comparative studies between non-asthmatic and asthmatic groups. In addition, some limitations are associated with bronchial cell monolayers, particularly because heterotypic cellular interactions are difficult to reproduce under these culture conditions. Over the last decade, bioengineering has enlarged the possibilities to develop tissue models (Langer and Vacanti, 1993) by combining the conventional cell culture ap- proaches to a concept according to which most cells can adopt specific three-dimensional orientation and organi- zation in an extracellular matrix, in response to proper stimuli induced mechanically in vitro. This concept has been confirmed in various bioengineered tissues produced in culture, notably in skin, blood vessels and ligaments (Bellows et al., 1982; Bouvard et al., 1992; Lopez-Valle et al., 1992; L’Heureux et al., 1993; Auger et al., 1995; TISSUE-ENGINEERED HUMAN ASTHMATIC BRONCHIAL EQUIVALENTS Jean-Sébastien Paquette, Véronique Moulin, Pierrot Tremblay, Vincent Bernier, Michel Boutet, Michel Laviolette, François A. Auger, Louis-Philippe Boulet and Francine Goulet Laboratoire de Génie Tissulaire/LOEX, Hôpital de l’Enfant-Jésus du CHA and Unité de Recherche en Pneumologie, Hôpital Laval, Québec, Canada. Materials Sciences and Applications, 2009, 1, 17-26  Human bronchial cell isolation The human bronchial cells were isolated by a new enzymatic technique and amplified in culture according to methods developed in our laboratory (Goulet et al., 1996a). Briefly, the processing was performed within 2-3 hours following the bronchoscopy. Collagen being the major constituent of bronchi matrix, collagenase was chosen to digest the col- lagen matrix of the biopsies. The human bronchial biop- sies were digested in 0.1% (0.2 U/ml) collagenase H (Boehringer Mannheim, Montreal, Canada) prepared in Dulbecco-Vogt modification of Eagles medium (DMEM) culture medium containing 10mM CaCl2 without any sup- plement. Tissues were digested overnight at 4°C because a collagenase digestion performed overnight at 37°C would reduce cell yield and viability. Homogenates were centri- fuged for 10 min at 300 g and the cell pellets were resuspended in DMEM supplemented with 10% fetal calf serum (FCS). All cells were plated in several 35-mm Petri dishes. The cultures were monitored daily and the dishes contain- ing epithelial cells were selected for addition of some le- thally irradiated 3T3 feeder cells (like it is done for skin epithelial cells, see Green et al., 1979). This technique al- lows the selection of pure epithelial cell populations (Goulet et al., 1996a). After 8-12 days in culture, bron- chial epithelial cells had reached 85% confluency and were ready to be stored and subcultured. The bronchial epithe- lial cells were cultured according to the method established for human keratinocytes (Germain et al., 1993), originally described by Green et al. (1979), in a combination of DMEM with Ham’s F12 in a 3:1 proportion (Gibco BRL, Burlington, Canada), supplemented with 24.3 µg/ml ad- enine (Sigma Chemicals, St-Louis, MO), 10 ng/ml human epidermal growth factor (EGF, Austral Biological, San Ramon, CA), 5 µg/ml bovine crystallized insulin, 5 µg/ml human transferrin (Boehringer Mannheim, Laval, Canada), 2x10-9 M 3,3',5', triiodo-L-thyronin (Sigma), 0.4 mg/ml hydrocortisone (Calbiochem, La Jolla, CA), 10-10 M chol- era toxin (ICN Biochemicals, Montreal, Canada), 10% re- constituted newborn calf serum (Immunocorp Sciences inc., Montreal, Canada) 100 IU/ml penicillin G and 25 µg/ml gentamicin (Sigma). Bronchial fibroblasts were obtained in the dishes which did not contain epithelial cells. They were cultured in DMEM supplemented with 10% fetal calf serum, 100 IU/ml penicillin G and 25 µg/ml gentamicin. They also reached 85% confluency after a week. The cul- ture media was changed three times a week. All cultures were kept in an 8% CO2 atmosphere at 37°C. Human bronchial cell viability The viability of epithelial cells over 3-4 passages and of fibroblasts over 8-9 passages, were comparable between asthmatic and non-asthmatic cell populations (over 85% viability). However, the yields of epithelial cells obtained from asthmatic biopsies were about 60% lower in primary culture, compared to non-asthmatic biopsies. That is ex- pected, since the epithelia of the asthmatic biopsies are of- ten poorer in cells and sometimes disorganized. Moreover, considering that we isolated the cells from 6-10 microbiopsies (1-2 mm-diameter), we believe that our Goulet et al., 2000; Chakir et al., 2001; Paquette et al., 2003). Thus, we have used several populations of the human bronchial cells isolated in our laboratory (Goulet et al., 1996a) from bronchial biopsies of asthmatic and non-asth- matic subjects, in an attempt to produce three-dimensional bronchial equivalents (BE) in vitro. For the first time, a bilayered BE containing human bronchial epithelial (HBEC) and fibroblastic cells (HBFC) isolated from asthmatic bi- opsies was obtained and maintained in culture for at least 15 days. An asthmatic HBEC layer grown on a mesenchy- mal counterpart seeded with asthmatic HBFC formed a BE referred to as asthmatic BE (a/aBE). Similarly, the non-asth- matic BE (na/naBE) was produced by combining non-asth- matic HBEC and HBFC in their respective layers. The in- teresting results from comparative analyses of these BEs are described in the present report. Materials and Methods Subjects Non-smoking asthmatic and normal subjects aged from 20 to 45 years, evaluated at the Laval Hospital asthma clinic, were enrolled in the study. The study was approved by the local Ethics Committee and subjects had given informed written consent. The normal subjects had a PC20 value (metacholine provocation, see below) over 16 mg/ml. All asthmatic subjects had a diagnosis of asthma according to the American Thoracic Society criteria (American Thoracic Society, 1987). All were atopic with at least one positive response to common allergens on allergy skin prick tests. Their asthma required only an inhaled β2 agonist agent on demand. None of the subjects reported a respiratory infec- tion or an increase in asthma symptoms in the month pre- ceding the study. They were not currently exposed to aller- gens to which they were sensitized apart from house-dust. Spirometry and response to inhaled methacholine were measured according to standardized procedures using aero- sols generated with a Wright’s nebulizer at tidal breathing for periods of 2 minutes (output = 0.13 ml/min) (Juniper et al., 1991). The provocative concentration of methacholine inducing a 20% fall in FEV1, the PC20, was determined. Skin prick tests were performed with a battery of common airborne allergens. Atopy was considered to be present if there was one or more positive response (> 3 mm wheal) to the inhalant allergens, with a positive reaction to histamine phosphate but not the diluent. Bronchoscopy and bronchial biopsies Before the bronchoscopy, a 200 µg dose of salbutamol was given using a metered-dose inhaler. All subjects received oxygen at 5 l / min by nasal catheter during bronchoscopy. After local anaesthesia of the throat, larynx and bronchi with 2% and 4% xylocaine, the flexible bronchoscope (Olym- pus OES 10 fiberscope, Olympus, Markham, Canada) was introduced into the bronchial tree and ten bronchial biop- sies were taken from the carinae of the right upper and mid- dle lobes and the segmental bronchi of the upper and lower lobes on both sides using conventional forceps. Vital signs, electrocardiograph and oximetry were recorded through- out the procedure. 18 J. S. Paquette et al.  method can be considered as successful (Goulet et al., 1996a). Human bronchial cell markers The respective morphological features of each cell type are very different and were confirmed by specific immunolabeling. Epithelial cells were immunolabeled with anti-keratin antibodies and fibroblasts were labeled with anti- vimentin antibodies (Goulet et al., 1996a). Production of three-dimensional human bronchial equivalents (BEs) Step 1: Preparation of the mesenchymal layer of the BEs. The BEs were produced according to the method pub- lished by Auger et al. (1995), with a few modifications. Briefly, a mixture of bovine Type I collagen (1.5 mg/ml) was prepared by dissolving the powder overnight at 4°C in sterile 0.017 M acetic acid. A solution of 0.84 ml of DMEM 2.7X containing 200 IU/ml penicillin G and 50 µg/ml of gen- tamicin, pH 8.0, was mixed with a second solution contain- ing 0.56 ml of FCS, 1.43 ml of the stock collagen solution, 30 µl of NaOH 0.7N and 0.15 ml of a HBFC suspension (1 X 106 cells/ml). This mixture was quickly distributed in a bacteriological Petri dish (35-mm diameter) already contain- ing the peripheral anchorage (sterile ring of Whatman pa- per), to produce the mesenchymal layers of each BE. The anchorage method, which prevents collagen lattice contrac- tion by the cells, was used as described previously (L’Heureux et al., 1993; Auger et al., 1995). Non-asthmatic (na) and asth- matic (a) HBFC were seeded individually in different mes- enchymal layers to produce naBE and aBE, respectively. The mesenchymal layers of the BE were covered with 2 ml of DMEM supplemented with 10% FCS, 100 IU/ml penicillin G and 25 mg/ml gentamicin following collagen polymeriza- tion and cultured in this medium until their epithelialization. Step 2: Epithelialization of the BE under submerged culture conditions. Four days later, the epithelialization was performed by seeding HBEC (8x105 cells / BE) on the mes- enchymal equivalents, maintained under submerged culture conditions until a confluent epithelial cell layer was obtained. Again, non-asthmatic and asthmatic HBEC were seeded on the corresponding mesenchymal counterparts. In some groups of BEs, HBEC from non-asthmatic source were seeded on mesenchymal layers containing asthmatic HBFC and vice versa. During the first 3 days after epithelia1ization, all BEs were cultured in the medium used for the culture of HBEC (see the section Human bronchial cell isolation). On the fourth day after epithelialization, the BEs were cultured in serum- free medium, supplemented with 24.3 mg/ml adenine, 10 ng/ ml human EGF, 5 mg/ml bovine crystallized insulin, 5 mg/ ml human transferrin, 2x10-9 M 3,3',5', triiodo-L-thyronin, 0.4 mg/ml hydrocortisone, 10-10 M cholera toxin, and 5x10-8 M retinoic acid (RA). The culture media were changed daily. Step 3: Culture at the air-liquid interface: The BEs were raised at the air-liquid interface as soon as a confluent layer of HBEC had covered their mesenchymal layers (6-8 days after epithelialization, depending on cell growth rates that could slightly vary from one experiment to another). The BEs were placed on Petri dishes containing an internal elevated support (Falcon No 3037). They were cultured at the air- liquid interface for equal number of days (a period varying from 5 to 7 days, according to each experiment), in the same media used under submerged conditions, but in the absence of EGF and cholera toxin since these growth factors enhance the production of gelatinases by the cells, which degrade the collagen matrix of the mesenchymal layer in culture at the air-liquid interface (Auger et al., 1995). The culture media were changed daily. Each ex- periment was done 3 times on at least 3 samples per group of BEs tested. Conditioned medium The conditioned medium (CM) was obtained from con- fluent non-asthmatic HBFC populations. The cells were grown until they had reached 95% confluence in a 75 cm2 Falcon culture dish. During the conditioning period of 7 hrs, the HBFC were kept in 12 ml of the same se- rum-free medium, used to culture the BEs under sub- merged conditions. Each sample of fresh CM was passed through a 0.22 µm Millipore low-binding protein filter in order to eliminate all cellular fragments before being transferred on the various groups of BEs. Fresh CM was produced and used daily, without any dilution. Histological analysis All BEs were fixed with Bouin’s solution and then em- bedded in paraffin. The 4 µm thick sections were stained using two different methods: the hematoxylin, phloxine and saffron staining and the PAS staining. To eliminate cross-reaction with endogenous glycogen in situ, some BE sections were digested before PAS staining with a solution of 0.5% maltase (Fisher) in phosphate-buffered saline (PBS) for 30 min at 37°C and washed 10 min with distilled water. At least 10 tissue sections were analyzed for each BE tested. We observed the entire sections to take representative pictures. Electron microscopy Some BEs were also processed and mounted for trans- mission and scanning electron microscope analyses as pre- viously described (Auger et al., 1993). Briefly, the sam- ples were fixed with a solution of 2% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.5 for 24 hrs, post- fixed with 1% osmium tetroxide, dehydrated with etha- nol and coated with gold (Sputtercoater, Nanotech, Man- chester, U.K.). Micrographs were made using Polaroid Polapan 400. A JEOL JSM-35CF scanning electron mi- croscope was used. Gelatinase activity assay The active gelatinases secreted in the BE culture media were semi-quantitatively analyzed by zymography, accord- ing to the method described previously (Auger et al., 1995). Briefly, one sample of all BE culture supernatants were collected daily, filtered and immediately frozen at - 70°C until use. At least 5 samples collected for each BE, during culture at the air-liquid interface, were analyzed by zymography. A constant volume (25 µl) of each sam- ple diluted in equal volume of 2x sample buffer was re- solved under non-reducing conditions by SDS-PAGE (Laemmli, 1970). The gels were rinsed and equilibrated 19 J. S. Paquette et al.  Figure 1. Histological analyses of different bilayered BE cultured for 6 days at the air-liquid interface in serum-free medium supplemented with RA. Masson trichrome staining of a BE produced with both non-asthmatic HBEC and HBFC (A), non-asthmatic HBEC and asthmatic HBFC (B-E), both asthmatic HBEC and HBFC (F-H), and asth- matic HBEC and non-asthmatic HBFC (I). The dashed line indicates the border of the mesenchymal layers in A, D- E, H-I. Note the holes between the epithelial and the mesenchymal layers (arrows) and the cell debris under the basement membrane (arrowheads) of the BEs produced with asthmatic HBEC (F-I). Scale bar 36µm. 20 J. S. Paquette et al.  in the proper buffer systems and put overnight at 37°C in digestion buffer (50 mM Tris, pH 7.4, containing 10 mM CaCl2 and 100 mM NaCl), under slow agitation. The gels were fixed with a 30% methanol solution containing 10% acetic acid and stained with 0.05% Coomassie blue pre- pared in the fixative. The gels were photographed and scanned. Results Histological analysis of BEs Histological analyses were performed on 4 different groups of BEs cultured for 6 days at the air-liquid interface. One of the advantages of our bilayered BE is the possibility to produce different types of bioengineered tissues, contain- ing HBEC and HBFC isolated from the same biopsies or from biopsies of different subjects (non-asthmatic or asth- matic). Thus, to evaluate and compare the growth of non- asthmatic and asthmatic HBEC on mesenchymal layers containing asthmatic and non-asthmatic HBFC, the four types of BEs were produced and analyzed. The first group of BEs was produced using both non- asthmatic HBEC and HBFC, na/naBEs. Masson trichrome staining of a na/naBEs is shown in Figure lA. The second group of BEs was produced by seeding non-asthmatic HBEC on a mesenchymal layer containing asthmatic HBFC, na/aBEs (Fig. 1, B-E). The third group of BEs was pro- duced using both asthmatic HBEC and HBFC, a/aBEs (Fig. 1, F-H). In the fourth group of BEs, asthmatic HBEC were grown on a mesenchymal layer seeded with non-asthmatic HBFC, a/naBEs (Fig. 1I). The thickness of the mesenchymal layers of the differ- ent BE groups showed differences upon culture time. As shown in figure 1, the mesenchymal layers of some a/aBEs (Fig. 1, F-G) and na/aBEs (Fig. 1, B-C) were degraded more slowly at the air-liquid interface, than the mesenchymal counterpart of na/naBEs (Fig. lA), and depending on the asthmatic HBFC population seeded in the different groups of a/aBEs and na/aBEs. The non-asthmatic HBEC reached a confluent state on the mesenchymal layers populated with non-asthmatic and asthmatic fibroblasts: na/naBEs (Fig. 1A) and na/aBEs (Fig. 1, B-E), respectively. However, these cells covered the mesenchymal layer of aBEs one day later than those seeded in naBEs (data not shown). Similarly, HBEC isolated from asthmatic subjects grew more slowly (in 8 days under sub- merged culture conditions) on all mesenchymal layers, Figure 2. Macroscopic view of rhodanile red stained BEs (A and B). Non-asthmatic (A) and asthmatic (B) HBEC were grown on a BE mesenchymal counterpart containing non-asthmatic HBFC in serum-free medium supple- mented with RA for 8 days under submerged culture conditions. Photomicrograph taken under phase constrast microscopy of BE sections after 18 days under the same culture conditions (C and D). Note the confluent layer of non-asthmatic HBEC (C), compared to the compact colonies (arrowheads indicate their borders) of asthmatic HBEC (D) slowly detaching from the mesenchymal counterpart. Scale bars: A-B, 0.5cm; C-D, 9 µm. 21 J. S. Paquette et al. 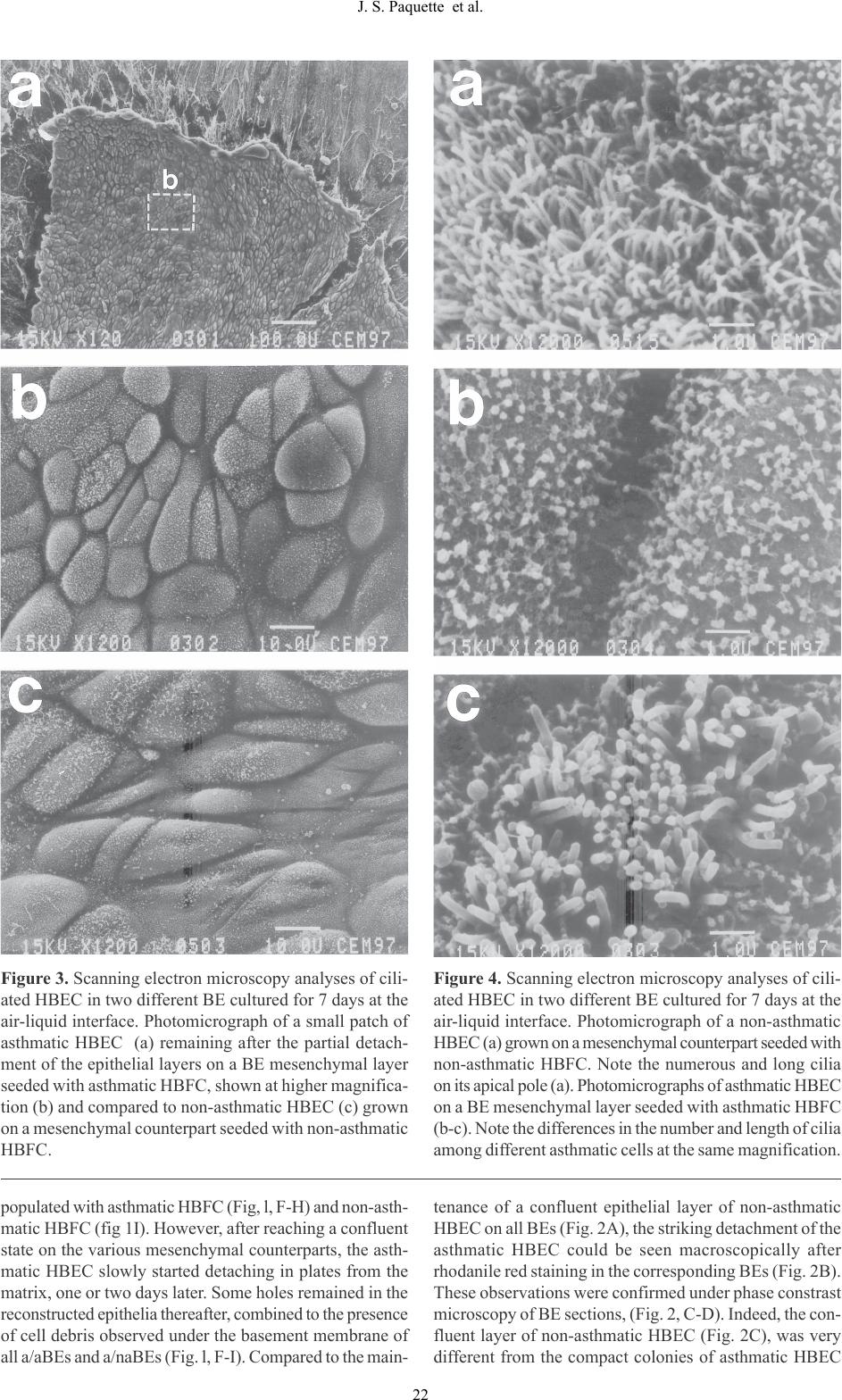 populated with asthmatic HBFC (Fig, l, F-H) and non-asth- matic HBFC (fig 1I). However, after reaching a confluent state on the various mesenchymal counterparts, the asth- matic HBEC slowly started detaching in plates from the matrix, one or two days later. Some holes remained in the reconstructed epithelia thereafter, combined to the presence of cell debris observed under the basement membrane of all a/aBEs and a/naBEs (Fig. l, F-I). Compared to the main- tenance of a confluent epithelial layer of non-asthmatic HBEC on all BEs (Fig. 2A), the striking detachment of the asthmatic HBEC could be seen macroscopically after rhodanile red staining in the corresponding BEs (Fig. 2B). These observations were confirmed under phase constrast microscopy of BE sections, (Fig. 2, C-D). Indeed, the con- fluent layer of non-asthmatic HBEC (Fig. 2C), was very different from the compact colonies of asthmatic HBEC Figure 3. Scanning electron microscopy analyses of cili- ated HBEC in two different BE cultured for 7 days at the air-liquid interface. Photomicrograph of a small patch of asthmatic HBEC (a) remaining after the partial detach- ment of the epithelial layers on a BE mesenchymal layer seeded with asthmatic HBFC, shown at higher magnifica- tion (b) and compared to non-asthmatic HBEC (c) grown on a mesenchymal counterpart seeded with non-asthmatic HBFC. Figure 4. Scanning electron microscopy analyses of cili- ated HBEC in two different BE cultured for 7 days at the air-liquid interface. Photomicrograph of a non-asthmatic HBEC (a) grown on a mesenchymal counterpart seeded with non-asthmatic HBFC. Note the numerous and long cilia on its apical pole (a). Photomicrographs of asthmatic HBEC on a BE mesenchymal layer seeded with asthmatic HBFC (b-c). Note the differences in the number and length of cilia among different asthmatic cells at the same magnification. 22 J. S. Paquette et al. 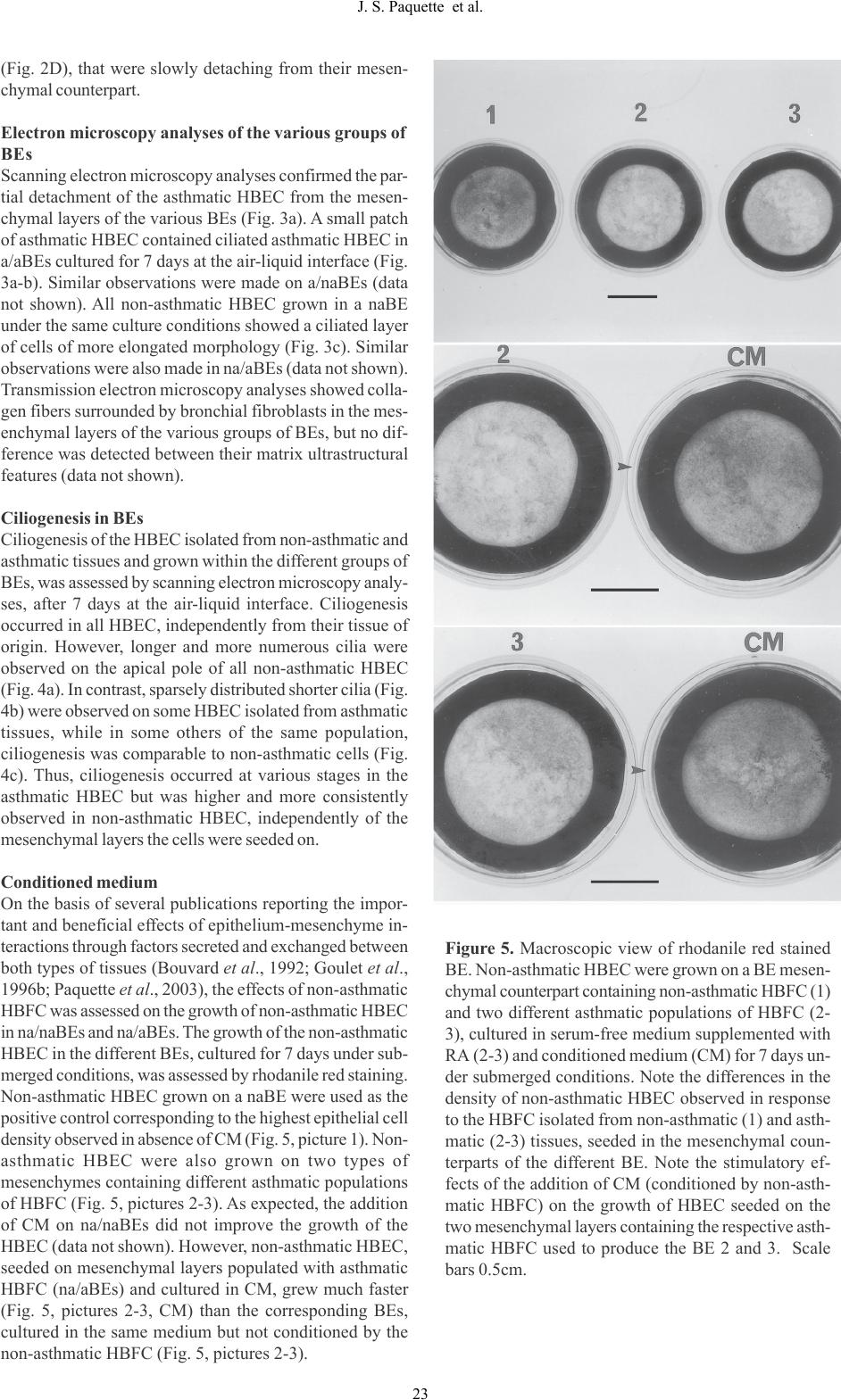 Figure 5. Macroscopic view of rhodanile red stained BE. Non-asthmatic HBEC were grown on a BE mesen- chymal counterpart containing non-asthmatic HBFC (1) and two different asthmatic populations of HBFC (2- 3), cultured in serum-free medium supplemented with RA (2-3) and conditioned medium (CM) for 7 days un- der submerged conditions. Note the differences in the density of non-asthmatic HBEC observed in response to the HBFC isolated from non-asthmatic (1) and asth- matic (2-3) tissues, seeded in the mesenchymal coun- terparts of the different BE. Note the stimulatory ef- fects of the addition of CM (conditioned by non-asth- matic HBFC) on the growth of HBEC seeded on the two mesenchymal layers containing the respective asth- matic HBFC used to produce the BE 2 and 3. Scale bars 0.5cm. (Fig. 2D), that were slowly detaching from their mesen- chymal counterpart. Electron microscopy analyses of the various groups of BEs Scanning electron microscopy analyses confirmed the par- tial detachment of the asthmatic HBEC from the mesen- chymal layers of the various BEs (Fig. 3a). A small patch of asthmatic HBEC contained ciliated asthmatic HBEC in a/aBEs cultured for 7 days at the air-liquid interface (Fig. 3a-b). Similar observations were made on a/naBEs (data not shown). All non-asthmatic HBEC grown in a naBE under the same culture conditions showed a ciliated layer of cells of more elongated morphology (Fig. 3c). Similar observations were also made in na/aBEs (data not shown). Transmission electron microscopy analyses showed colla- gen fibers surrounded by bronchial fibroblasts in the mes- enchymal layers of the various groups of BEs, but no dif- ference was detected between their matrix ultrastructural features (data not shown). Ciliogenesis in BEs Ciliogenesis of the HBEC isolated from non-asthmatic and asthmatic tissues and grown within the different groups of BEs, was assessed by scanning electron microscopy analy- ses, after 7 days at the air-liquid interface. Ciliogenesis occurred in all HBEC, independently from their tissue of origin. However, longer and more numerous cilia were observed on the apical pole of all non-asthmatic HBEC (Fig. 4a). In contrast, sparsely distributed shorter cilia (Fig. 4b) were observed on some HBEC isolated from asthmatic tissues, while in some others of the same population, ciliogenesis was comparable to non-asthmatic cells (Fig. 4c). Thus, ciliogenesis occurred at various stages in the asthmatic HBEC but was higher and more consistently observed in non-asthmatic HBEC, independently of the mesenchymal layers the cells were seeded on. Conditioned medium On the basis of several publications reporting the impor- tant and beneficial effects of epithelium-mesenchyme in- teractions through factors secreted and exchanged between both types of tissues (Bouvard et al., 1992; Goulet et al., 1996b; Paquette et al., 2003), the effects of non-asthmatic HBFC was assessed on the growth of non-asthmatic HBEC in na/naBEs and na/aBEs. The growth of the non-asthmatic HBEC in the different BEs, cultured for 7 days under sub- merged conditions, was assessed by rhodanile red staining. Non-asthmatic HBEC grown on a naBE were used as the positive control corresponding to the highest epithelial cell density observed in absence of CM (Fig. 5, picture 1). Non- asthmatic HBEC were also grown on two types of mesenchymes containing different asthmatic populations of HBFC (Fig. 5, pictures 2-3). As expected, the addition of CM on na/naBEs did not improve the growth of the HBEC (data not shown). However, non-asthmatic HBEC, seeded on mesenchymal layers populated with asthmatic HBFC (na/aBEs) and cultured in CM, grew much faster (Fig. 5, pictures 2-3, CM) than the corresponding BEs, cultured in the same medium but not conditioned by the non-asthmatic HBFC (Fig. 5, pictures 2-3). 23 J. S. Paquette et al.  Gelatinases secretion by human bronchial cells Gelatinases A, zymogen of MMP-2 (proMMP-2, 72 kDa), and activated MMP-2 (active MMP-2, 62.5 kDa) and B (MMP-9, 92 kDa) were recently described as secretion prod- ucts of HBFC and HBEC, respectively (Hoshino et al., 1998). The gelatinases secreted by the BEs cultured at the air-liquid interface were analyzed by zymography. At least 5 different samples were investigated for each BE tested and we show in Fig. 6 a representative zymogram. Our re- sults showed that the active form of gelatinase A (62.5 kDa) was secreted by all HBFC in the BE supernatants (Fig. 6, lanes 1-4), including the supernatant of a/aBEs cultured in presence of CM (Fig.6, lane 4). The gelatinase B (92 kDa) was also actively secreted by the various HBEC in all BE supernatants (Fig, 6, lanes 1-4), but the zymograms showed slightly lower activities of this enzyme in the supernatants taken from a/aBEs (Fig. 6, lane 2-4), compared to na/naBEs (Fig. 6, lane 1). Interestingly, the sample that contained the lowest amounts of gelatinase A (72 kDa), associated with fibroblast secretion products, corresponded to the culture supernatant resolved in lane 3 (Fig. 6), collected from the a/aBE. This BE showed the thicker mesenchymal layer on histological sections (Fig. 1F), compared to the other BEs cultured for 6 days at the air-liquid interface. This sample (Fig. 6, lane 3), was compared to the supernatant of another a/aBE, pro- duced with the same cells, but cultured in presence of me- dium conditioned by non-asthmatic HBFC (Fig. 6, lane 4). The CM-stimulated-a/aBE (Fig. 6, lane 4), contained more of the precursor form of the gelatinase A (72 kDa). It also contained less of the active 62.5 kDa gelatinase than the other BEs (Fig. 6, lanes 1-3). However, this a/aBE showed a slightly thinner mesenchymal layer on histological analy- ses than the na/naBEs (Fig. 1A). Thus, it is difficult to es- tablish a correlation between the amounts of gelatinases secreted in the culture supernatants of the various BEs and the rate of degradation of their mesenchymal layers in cul- ture. Other types of proteases may be involved in this proc- ess. Figure 6. Zymograms (top) and corresponding SDS-PAGE (bottom) showing the relative gelatinase activities analyzed from supernatants of naBE (lane 1), of two different a/aBEs (lanes 2 and 3) and of an a/aBE cultured in the medium conditioned by HBFC (lane 4). The arrowheads indicate the gelatinase A (MMP-2, 72 kDa). The arrows indicate the gelatinase B (MMP-9, 92Kda) and the active form of gelatinase A (62.5 kDa). 24 J. S. Paquette et al.  Discussion The production of bioengineered BEs with non-asthmatic and asthmatic cells allowed the comparative analyses of severa1 histologic and functional parameters in vitro. Our results suggest that HBEC isolated from asthmatic sub- jects can grow on a mesenchymal layer seeded with non- asthmatic and asthmatic HBFC. However, if they grow bet- ter on a non-asthmatic than on an asthmatic type of mesen- chymal layer, they all started to detach from their mesen- chymal support one to two days after reaching confluence. Such process continued thereafter, under submerged cul- ture conditions and subsequently, at the air-liquid interface. In contrast, the non-asthmatic HBEC never detached from any type of mesenchymal layer tested. When asthmatic HBEC are grown in monolayers on plastic, they can be maintained in culture for at least 3-4 passages without de- taching from the culture dishes, in a medium supplemented with serum. When grown in BEs, their detachment from the various mesenchymal layers, correlates with the for- mation of holes and the deposition of cell debris at the in- terface epithelium-mesenchyme. These data suggest that asthmatic HBEC fail to maintain stable points of anchor- age with the matrix support, probably due to an absence or a loss of membranous anchorage proteins or receptors. In absence of serum that contains several growth factors and fibronectin, such defect may be observed earlier. We may believe that the behavior of the HBEC isolated from asth- matic subjects seeded on BEs is comparable to the obser- vations reported in asthmatic tissues in vivo when desqua- mation occurs (Jeffery et al., 1989). Ciliogenesis was observed, at least partially, in all groups of BEs. However, several asthmatic HBEC did not show cilia as numerous and/or as long as the non-asthmatic HBEC. These data suggest that the asthmatic HBEC do not reach the same level of differentiation within a given population isolated from the same tissues. Such observa- tions were also reported from histologic analyses of severe asthmatic bronchi, whereas several non-ciliated cells were detected at the surface of their epithelium (Jeffery et al., 1989). Again, it was not surprising to observe this phe- nomena in our BE in vitro. The non-asthmatic HBEC grew more slowly within a na/aBE and the histologic organization of the na/aBEs was less compact than in the na/naBEs. Inversely, the asthmatic HBEC grew faster on a mesenchymal layer containing non- asthmatic HBFC than in a aBE. These data strongly sug- gest that the HBEC respond to some factors secreted by the HBFC up to a certain degree, and may as well release other factors to the mesenchymal cells. It was previously demon- strated in vitro that epithelial cells are an important regula- tor of airway remodeling by means of paracrine control of bronchial fibroblasts (Zhang et al., 1999). Other evidence supporting the existence of epithelium-mesenchyme inter- actions in the BEs is the positive effect of the CM col- lected from non-asthmatic HBFC on the growth of non- asthmatic HBEC on a mesenchymal layer seeded with asth- matic HBFC. It seems that the CM contains cytokines that could compensate a lack of factors secreted by the asth- matic HBFC in the na/aBE. The comparative analyses of the active gelatinases se- creted in the BEs culture supernatants showed some dif- ferences, but the 92 kDa and the 62.5 kDa gelatinases were detected in all BE supernatants. The supernatant of the a/ aBE showing the thicker mesenchymal layer may be ex- plained by a lower secretion of gelatinase A (72 kDa) or by the synthesis of other collagen types, such as Type V (Hoshino et al., 1998). The apparent sub-epithelial fibro- sis associated with asthma is postulated to be caused by fibroblasts. These cells are involved in collagen synthesis and remodelling and their functional status may be modu- lated by cytokines secreted by inflammatory or epithelial cells (Brewster et al., 1990; Roche, 1991; Gauldie et al., 1992). Thus, several hypotheses could be considered to explain our results. The zymograms on gelatin, a substrate rich in collagen Type I, do not reveal several other collagenases and proteases that may be secreted in differ- ent amounts by asthmatic and non-asthmatic cells present in our different BEs. It would be interesting to assess the effects of specific inhibitors of MMP-9 and MMP-2 on the maintenance of the mesenchymal layers of the different groups of BEs. Taken together, we conclude that the tissue-engineered human BE is a good model to investigate cellular mecha- nisms involved in bronchial disorders like asthma. This approach allows the analyses of several parameters such as the bronchial cell growth and differentiation, their organi- zation and their secretion products in vitro. Comparative toxicological and pharmacological studies of various hu- man bronchial cell populations can be achieved in vitro, using BE as a precious alternative to animal use. Eventu- ally, other cell types, such as immune or smooth muscle cells could be added to the model to investigate more com- plex interactions. Acknowledgements The authors are grateful to Dr Hilda López Valle, M.D., pathologist, for her critical analyses of the histological and ultrastructural properties of the bronchial equivalents, Mr. Aristide Pusterla for his contribution in electron microscopy analyses, Mr. Claude Marin for photographic assistance, Mrs Nathalie Tremblay for her technical assistance on this project. Mr. Jean-Sébastien Paquette was recipient from the NSERC fellowship and a honorary fellowship from Laval University. Drs F. Goulet, F.A. Auger, and L.-P. Boulet were recipients of Scolarships from the Fonds de la Recherche en Santé du Québec. References American Thoracic Society (1987) Standards for the di- agnosis and care of patients with chronic obstructive pul- monary disease (COPD) and asthma. Am Rev Respir Dis 136: 225-244. Auger FA, Guignard R, Lopez Valle CA, Germain L (1993) Role and inocuity of Tisseel®, a tissue glue, in the grafting process and in vivo evolution of human cultured 25 J. S. Paquette et al.  epidermis. Br J Plastic Surg 46: 136-142.29. Auger FA, Lopez Valle CA, Guignard R, Tremblay N, Noël B, Goulet F, Germain L (1995). Skin equivalents pro- duced using human collagens. In Vitro Cell Dev Biol 31: 432-439. Baeza-Squiban A, Boisvieux-Ulrich E, Guilianelli C, Houcine O, Geraud G, Guennou C, Marano F (1994) Ex- tracellular matrix-dependent differentiation of rabbit tra- cheal epithelial cells in primary culture. In Vitro Cell Dev Biol Anim 30A: 56-67. Bellows CG, Melcher AH, Aubin JE (1982) Associa- tion between tension and orientation of periodontal liga- ment fibroblasts and exogenous collagen fibres in collagen gels in vitro. J Cell Sci 58: 125-138. Boulet LP, Turcotte A, Boutet M, Milot J, Côté J, Malo JL, Cartier A, Dugas M, Laviolette M (1993) Correlations between airway inflammation and responsiveness to metacholine in subjects with asthma, rhinitis, reactive air- ways dysfunction syndrome (RADS), chronic laugh and normals. Eur Respir J 6(S17): 265S (abstract). Bouvard V, Germain L, Rompré P, Roy B, Auger FA (1992). Influence of dermal equivalent maturation on a skin equivalent development. Biochem Cell Biol 70: 34-42. Brewster CEP, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR (1990) Myofibroblasts and sub- epithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 3: 507-511. Chakir J, Page N, Hamid Q, Laviolette M, Boulet LP, Rouabhia M (2001) Bronchial mucosa produced by tissue engineering: a new tool to study cellular interactions in asthma. J Allergy Clin Immunol 107: 36-40. Chung KF (1996) Effects of nedocromil sodium on air- way neurogenic mechanisms. J Allergy Clin Immunol 98: S112-S117. Davenport EA, Nettesheim P (1996) Regulation of mucoci1iary differentiation of rat tracheal epithelial cells by type 1 collagen gel substratum. Am J Respir Cell Mol Biol 14: 19-26. De Jong PM, Van Sterkenburg MAJA, Hesseling SC, Kempenaar JA, Mulder AA, Mommaas AM, Dijkman JH, Ponec M (1994) Ciliogenesis in human bronchial epithe- lial cells cultured at the air-liquid interface. Am J Respir Cell Mol Biol 10: 271-277. DiCosmo B, Goba G, Picarella D, Elias JA, Rankin JA, Stripp B, Whitset JA, Flavell RA (1995). Expression of interleukin-6 by airway epithelial cells. Effects on airway inflammation and hyperreactivity in transgenic mice. Chest 107: 131S (abstract). Djukanovic R, Roche WR, Wilson JW, Beasley CRW, Twentyman OP, Howarth PH, Holgate ST (1990) Mucosal inflammation in asthma. Am Rev Respir Dis 142: 434-457. Gauldie J, Jordana N, Cox G, Ohtoshi T, Dolovich J, Denburg J (1992) Fibroblasts and other structural cells in airway inflammation. Am Rev Respir Dis 145: S14-S17. Germain L, Rouabhia M, Guignard R, Carrier L, Bouvard V, Auger FA (1993) Improvement of human keratinocyte isolation and culture using thermolysin. Burns 19: 99-104. Goulet F, Boulet LP, Chakir J, Tremblay N, Dubé J, Laviolette M, Auger FA (1996a). Morphological and func- tional properties of bronchial cells isolated from normal and asthmatic subjects. Am J Resp Cell Mol Biol 15, 312- 318. Goulet F, Poitras A, Rouabhia M, Cusson D, Germain L, Auger FA (1996b) Stimulation of human keratinocyte proliferation through growth factor exchanges with dermal fibroblasts in vitro. Burns 22: 107-112. Goulet F, Germain L, Rancourt D, Caron C, Normand A, Auger FA (2000) Tendons and ligaments. In: Principles of Tissue Engineering, 2nd ed. Lanza R, Langer R, Vacanti J (eds). Academic Press, San Diego, pp 711-722. Gray EG, Guzman K, Davis CW, Abdullah LH, Nettesheim P (1996) Mucoci1iary differentiation of seri- ally passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 14: 104-112. Green H, Kehinde O, Thomas J (1979) Growth of cul- tured human epidermal cells into multiple epithelia suit- able for grafting. Proc Natl Acad Sci (USA) 76: 5665-5668. Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S (1998) Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflamma- tion. J Allergy Clin Immunol 102: 783-788. Jeffery PK, Wardlaw AJ, Nelson FC, Collins JV, Kay AB (1989) Bronchial biopsies in asthma. Am Rev Respir Dis 140: 1745-1753. Juniper EF, Cockcroft DW, Hargreave FE (1991) Tidal breathing method. In Histamine and Methacholine Inhala- tion Tests: Laboratory Procedure and Standardization. Ca- nadian Thoracic Society. AB Draco, Lund, Sweden. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Na- ture 227: 680-685. Langer R, Vacanti JP (1993) Tissue engineering. Sci- ence 260: 920-926. L’Heureux N, Germain L, Labbé R, Auger FA (1993) In vitro construction of a human blood vessel from cul- tured vascular cells. J Vasc Surg 17: 499-509. Lopez Valle CA, Auger FA, Rompré P, Bouvard V, Germain L (1992) Peripheral anchorage of dermal equiva- lents. Br J Dermatol 127: 365-371. Opazo-Saez A, Pare PD (1994) Stimulus-response re- lationships for isotonic shortening and isometric tension generation in rabbit trachealis. J Appl Physiol 77: 1638- 1643. Paquette JS, Tremblay P, Bernier V, Auger FA, Laviolette M, Germain L, Boutet M, Boulet LP, Goulet F (2003) Pro- duction of tissue-engineered three-dimensional human bronchial models. In Vitro Cell Dev Biol-Animal 39: 213- 220. Roche WR (1991) Fibroblast and asthma. Clin Exp Al- lergy 21: 545-548. Shichinoke K, Shimizu M, Kurokawa K (1996) Effect of M-711 on experimental asthma in rats. J Vet Med Sci 58: 55-59. Widdicombe J (1996) The tracheobronchial vasculature: a possible role in asthma. Microcirculation 3: 129-141. Zhang S, Smartt H, Holgate ST, Roche WR (1999) Growth factors secreted by bronchial epithelial cells con- trol myofibroblast proliferation: an in vitro co-culture model of airway remodeling in asthma. Lab Invest 79: 395- 405. 26 J. S. Paquette et al. |

