Biomass Productivity and Fatty Acid Composition of Chlorella lobophora V M Andreyeva, a Potential Feed Stock for Biodiesel Production ()
1. Introduction
Biomass is considered to be one of the good alternatives for the current energy crisis. Algae are the fastest known producers of biomass per unit time, space and nutrient resources [1] . Rate of photosynthetic production in algae is higher than that of land plants [2] . Algae grow in a wide range of freshwater and wet soil habitats [3] . Highly ecologically favorable systems are available for economically viable production and cultivation of algae. Many algae have the ability to multiply and double their biomass from 3.5 hours to a full day [4] .
Biomass is either liquefied directly to fuels or the oil extracted from oil-yielding biomass is esterified to potential fuels such as bio-diesel. Algal biomass is not only a potential source of high-value bio-oils but also yields chemicals, foods, food additives, therapeutic materials and animal feeds [5] . Moreover, algal culture in waste- waters always ensure the recovery of lost nutrients [6] and during the culture water is cleaned by means of phycoremediation. Among microalgae Chlorella species are proved to be highly industrially valuable for the production of lipids and biomasses [7] , tolerant to high concentrations of CO2 [8] , adaptable to various environmental conditions [9] , useful in the treatment of industrial effluents [10] [11] and purification of waste-water systems [12] .
However, biomass productivity and accumulation of specific compounds such as oil in algae depend upon the environment conditions and media compositions [13] . Environmental conditions that influence algal productivity and oil yield include duration and quality of sunlight, temperature, relative humidity, evaporation, precipitation, topography of lands, nutrients, carbon sources and water qualities [14] . Quality and quantity of biomass and oil yield of algae also depends on the kind of species and strains from local environments [15] . During in vitro culture of algae, the factors that influence productivity include media composition and cultural conditions [16] . Factors in the medium of growth have direct influence on specific product accumulations in algae; especially algal fatty acid composition within the biomass [17] . Oils and nutraceutically important pigment accumulations in algae depend on their media components and pH conditions [18] [19] . Some species of algae require specific growth media for high biomass productivity and sufficient lipid accumulation [20] .
Among the specific growth media available for the mono-culturing of algae, Bold’s Basal Medium (BBM) is one of the best general media, which is already demonstrated as a good medium for the culture of Chlorella sp., especially for high amount of total protein and chlorophyll pigments in the biomass [21] . Glucose enrichment in BBM under nitrogen limitation yields higher amount of lipids in Chlorella sorokiniana Shihira & R. W. Krauss while no significant progress in the biomass productions [22] . In vitro experimental culture of specific algal species in specific media is therefore, inevitable to identify better species for biomass and other valuable resources, especially of oil yield from algae.
Screening of algal strains adapted to a wide range of environmental stress and the production of high biomass and lipids are of great importance in the current scenario. Several species of Chlorella from different climate zones are known for high quantity of lipids and biomass [23] . However, no reports are available on the biomass and oil-yield potentials of Chlorella lobophora, especially a local strain of the species that is quite common in bloomed water bodies of Kerala. The alga collected from a pond at Kasaragod District of Kerala is used in this study.
Major objectives of the present experimental study included comparison of in vitro biomass productivity of Chlorella lobophora in BBM and modified BBM; extract the algal oil from the biomass obtained from both media, chemical characterization of the oil, and feasibility of biodiesel production from the extracted oil. This is the pioneer report of biomass productivity and oil yield from Chlorella lobophora.
2. Materials and Methods
2.1. Collection and Isolations of Algal Strain
Freshwater sample containing local strains of Chlorella lobophora (Figure 1(a)) was collected in a sterilized one litre bottle from the bloomed freshwater pond of Ananthapura Temple (12˚35'03.2"N, 74˚58'55.4"E), Kasaragod District, Kerala, India during summer and rainy seasons. The pH of the water sample was measured and the alga collected was identified by using online algae database of Guri and Guri [24] . 50 mL of water sample were centrifuged at 6000 rpm at room temperature (27˚C - 30˚C) for 5 minutes to concentrate the algal cells of the sampled water. High magnification digital compound microscope (Motic BA 310) was used for observation of the algal species. Serial dilution of water sample followed by micro capillary isolation method [25] and streak
![]()
Figure 1. (a) Optical microscopic image of Chlorella lobophora; (b) Comparison of biomass production of alga in modified and normal Bold’s Basal medium.
plate method were used for the isolation of the alga in pure form. The isolated strain was cultured in Bold’s Basal Medium (BBM) at pH of 7.30. Pure culture of the strain in BBM is maintained in the algal culture facility centre in the Ecotechnology Laboratory, School of Biosciences, Mahatma Gandhi University.
2.2. Comparison of Biomass Productivity of the Alga
Cultures were carried out in one litre flasks using Bold’s Basal Medium [26] and modified Bold’s Basal Medium (Table 1) in triplicate (Figure 1(b)). All the culture vessels were incubated under controlled conditions of light (8000 Lux), temperature (24˚C ± 2˚C) and pH (7.30). Productivity was measured on completion of 30 days of growth. On completion of the incubation period the biomass was collected by centrifugation and the solid biomass was further air dried. Percentage increase of biomass per day per litre was calculated as productivity of algae, which is calculated by the formula

2.3. Algal Oil Extraction
Fresh algal biomass collected by centrifugation from the culture was oven dried (approximately at 40˚C for 24 hrs). Ten gram of oven dried biomass was taken into a round bottom flask and added 100 mL of chloroform: methanol (2:1 v/v) mixture into the biomass. The biomass was then kept soaked in the organic solvents at room temperature (27˚C - 30˚C) under continuous shaking at 150 rpm for 4 hrs in a rotary shaker; afterwards the mixture was centrifuged at 6000 rpm at room temperature for 5 minutes. Residual biomass was separated from the extract and then the oil along with the solvent was transferred in to a separating funnel. About 40 mL of distilled water was added to this mixture to separate the oil from the solvent. The oil got separated as an organic phase in bottom layers, which was then collected into a bottle. The separated biomass and the oil were made free of the solvent by using rotary evaporator.
The air dried residual biomass free of the solvent was further subjected to hot method of extraction for the collection of the remaining neutral lipids. The biomass was taken in to a Soxhlet extractor and extracted with 75 mL of hexane, refluxed under 70˚C for 2 hours. The extracted oil components were collected and the oil was made free of the solvent by using rotary evaporator. Finally, the two extracted oil samples were mixed together to get the total oil extract.
2.4. Chemical Characterization of Algal Oil
50 mg of algal oil was saponified with 1 mL of saturated KOH-CH3OH solution at 50˚C for 10 minutes and then followed by methanolysis with 5% HCl in methanol at 60˚C for another 10 minutes in screw capped test tubes. The methyl fatty acids were separated by adding 2 mL of water into it and fatty acid phase was recovered. GC-MS (Agilent make 7890A-5975C) instrument was used for the fatty acid profiling. 1 mL of methyl fatty acid sample was injected to the GC column. Helium was used as carrier gas at flow rate of 54 mL/min. Chromatographic data was recorded and compared using Agilent data analysis software.
![]()
Table 1. Chemical composition of bolds basal media and modified bolds basal media.
2.5. Trans Esterification of Algal Oil and the Production of Biodiesel
About 400 mg of algal oil extracted were taken into a round bottom flask and mixed with 15 mL of methanolic sulphuric acid containing 2% sulphuric acid in methanol (v/v) and refluxed at 60˚C for 4 hours with continuous shaking. The reaction was monitored by thin layer chromatography (TLC) with the solvent system, Hexane: Ethyl acetate/hexane: Toluene at the ratio of 9:1. The reaction was continued till the oil spot was disappeared on TLC plate. After the completion of reaction (2 - 4 hr), the contents were transferred to separating funnel and 25 mL water was added to it. The aqueous layer was extracted twice with ethyl acetate (25 mL each) and pooled the ethyl acetate layer. The extract was dried over anhydrous Na2SO4 and concentrated under vacuum.
2.6. FTIR Analysis of Algal Biodiesel
Thermo Scientific IS10 FTIR instrument was used for analyzing the algal biodiesel. The samples were analyzed in transmission mode in 400 - 4000 cm−1 wave number range.
2.7. Statistical Analysis
Biomass productivity is presented as means ± standard deviation of three replicates of algal samples in each medium; t-test is carried out to examine whether the difference in productivity of the species in two different media are significant.
2.8. Calculation of Fatty Acid Composition of Algal Oil
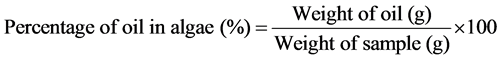
3. Result and Discussion
3.1. Biomass and Lipid Productivity of Alga
Details of the biomass and lipid productivity as percentage increase of biomass per day per litre are given in Table 2. There was a significant difference in the yield of biomass and lipid of the alga between the two media. Chlorella species in BBM medium produces higher amount of protein and chlorophyll, compared to other culture media [21] . In the present investigation, modified Bold’s Basal Medium with pH 7.30 showed higher biomass and lipid productivity of the alga than that in normal Bold’s Basal Medium. The oil yield of alga grown in normal BBM was found to be 28% of the total dried algal biomass, while the yield in the modified BBM was 35%. Statistical analysis has shown that biomass and lipid production of this alga in modified Bold’s Basal Medium is significantly higher than that in the normal Bold’s Basal Medium. Increase in biomass yield in accordance with change in medium compositions is already reported in another species of Chlorella [27] . However, in the current experiment using modified BBM, increase in yield of both biomass and lipid content observed for Chlorella lobophora in the modified medium. The nutrients added to modify the BBM are fertilizers such as urea, potassium chloride and ammonium phosphate, which are locally available and hence the modification is comparatively easier and affordable at commercial scales. Therefore, observations of this pilot experimental study on the suitability of a local strain of Chlorella lobophora as a potential crop for biomass and bio-oil has commercial feasibility. However, more experimentation of the same in diverse environmental conditions and nutrient conditions is required to standardize the specimen before commercial applications. Moreover, change in lipid quality in relation to environment conditions also need to be ascertained.
Chemical profile of the oil from this alga (Table 3) has shown that the major fatty acid component of the oil is Palmitic acid (C16:0), which accounts 27.2% of total fatty acids. Among all fatty acids, monosaturated fatty acids constitute 34.28% followed by 14.43% monounsaturated and 18% polyunsaturated fatty acids. Such an
![]()
Table 2. Biomass and lipid productivity in relation to environmental conditions in two different media.
algal feed stock rich in mono-saturated and mono-unsaturated fatty acids are desirable for biodiesel production [28] . The most commonly used fatty acids for biodiesel productions in relation to quality are containing C16:0 and C18:1 fatty acids [29] . According to the American Society for Testing and Materials (ASTM) D6751 and European EN 14214 standards, monosaturated fatty acids are given preferences for the production of good quality biodiesels [30] . Therefore, Chlorella lobophora, which is capable of providing more of monosaturated fatty acids than unsaturated fatty acids, is a good option for biofuel production. Moreover, as 18% of its total fatty acid is constituted by the three important essential fatty acids, it has nutraceutical value as well.
3.2. Trans Esterification of Algal Oil and FTIR Confirmation of Biodiesel
The oil extracted from the alga could successfully be converted to biodiesel using acid catalyzed transesterification method. The IR spectra (Figure 2) peak 1742.08 cm−1 of transesterified algal oil was obtained from FT-IR spectrometer indicating the formation of biodiesel. As the characteristic peaks of esters in FTIR spectrum due to C=O and C-O bond around 1750 - 1730 cm−1 [31] are seen in the spectra, it became clear that the product is a mono alkyl ester, biodiesel. The spectrum of methyl esters is generally showing the wave range of 1750 - 1730 cm−1 [32] , which is generally absent in petro-diesel spectra [33] .
![]()
Table 3. GCMS profile of algal fatty acid.
![]()
Figure 2. FTIR spectrum of algal biodiesel.
4. Conclusion
For Chlorella lobophora, Bold’s Basal Medium is found to be suitable for small scale production of the alga and also for maintaining culture stocks. Compared with BBM, modified Bold’s Basal Medium with little higher NPK provided as commercially available chemical fertilizers is found to be a better medium for higher yield of biomass and lipid from the alga. Oil content of Chlorella lobophora is 28% and 35% of its dry biomass in the BBM and modified BBM respectively. Fatty acid composition of the oil in the alga has significant percentage of monosaturated and monounsaturated fatty acids, which is suitable for biodiesel production. In adition to the biofuel applications, the oil is nutraceutically important because of 18% of essential fatty acid in the oil. Therefore, the local strain of Chlorella lobophora is found to be a promising species of alga for nutraceutical and biofuel feedstock. Further explorations on lipid production potential of this alga in different media and under different cultural conditions are continuing. Moreover, oil composition in relation to different cultural conditions also is getting standardized.
Acknowledgements
Support extended by Dr. I. Ibnusaud Director, Institute for Intensive Research in Basic Sciences, Mahatma Gandhi University for FTIR analysis carried out at his laboratory is gratefully acknowledged.
NOTES
*Corresponding author.