Biological activities of the hydro-alcoholic and aqueous extracts of Achillea fragrantissima (Forssk.) grown in Jordan ()
KEYWORDS
Achillea fragrantissima; Asteraceae; Antioxidant Activity; Antiplatelet Activity; Cytotoxicity; Jordan
1. INTRODUCTION
The genera Achillea (Family Asteraceae) with over 140 species are perennial herbs which are widely distributed in Southern European countries and in the Middle East [1]. Since antiquity, Achillea species have been used in traditional medicine of several civilizations to alley pain, spasms and inflammation. Numerous studies describe the ethnopharmacological value and various pharmacological effects of the Achillea extracts and hydrodistilled volatile oils in management of several diseases, used topically and orally [2-8]. Also in Jordan, Achillea species are used as herbal remedies against fever, common cold, digestive complaints, and as haemostatic topically for slow-healing wounds and skin inflammations [9]. Among the five Achillea species, A. falcata, A. fragrantissima, A. biberstennei, A. santolina and A. aleppico, reported to occur in Jordan, only A. aleppico is a rare plant, while the former four species are quiet abundant mainly in the Mediterranean and Irano-Turanian biogeographic zones of Jordan [10].
A. fragrantissima has been used for many years in traditional medicine in Middle Eastern countries for the treatment of respiratory diseases, skin diseases, gastrointestinal disturbances, high blood pressure, stomach aches and diabetes [11-14]. Recent reports demonstrated anti-inflammatory, antioxidant and antiproliferative capacities of A. fragrantissima extracts [15-17]. To the best of our knowledge, there are no comprehensive studies performed to evaluate the biological propensities of A. fragrantissima although its infusion is a preferred traditional medication countrywide. Hence, the objective of the present study was to screen the antioxidant, antiplatelet, antimicrobial and antiproliferative efficacy of A. fragrantissima grown wild in Jordan.
2. MATERIALS AND METHODS
2.1. Plant Material
A. fragrantissima was collected from Al Jubeiha region, a suburb of the capital Amman, during the flowering period late spring, 2011. The plant was identified by Prof. Barakat E. Abu-Irmaileh at the Department of Plant Protection, Faculty of Agriculture, The University of Jordan. Voucher specimen has been deposited in the Department of Pharmaceutical Sciences, Faculty of Pharmacy, The University of Jordan. Flowering aerial parts were air dried at room temperature (RT) in the shade for one week until constant weight, and subsequently used for extraction.
2.2. Preparation of Extracts
The extracts were prepared by refluxing each 10 g of the dried coarsely powdered plant material with 100 ml distilled water or with 70% ethanol until boiling and kept overnight. Subsequently, the extracts were filtered and evaporated in vacuo to give crude residues in the following yields (%, w/w): 27% and 21.5%, respectively.
2.3. Evaluation of Total Phenolic Compounds
From the crude extracts, stock solutions of 50 mg/ml (80% methanol (MeOH) or H2O) were prepared. Total phenolic content was determined using the Folin-Ciocalteu colorimetric modified method with gallic acid as standard [18]. Briefly, 50 μl aliquots from each of the replicates were mixed with 450 μl of distilled water and 2.5 ml of 0.2 N Folin-Ciocalteu reagent. After 5 min, 2 ml of saturated sodium carbonate (Na2CO3; 75 g·L−1) was added. The absorbance of the resulting blue solution was measured at 765 nm after incubation at 30˚C for 1.5 h with intermittent shaking. Quantitative measurements were performed based on a six point standard calibration curve of 20, 100, 200, 300, 400, 500 mg·L−1 of gallic acid in 80% MeOH. Total phenolic content is expressed as gallic acid equivalents (GAE) in milligrams per gram dry material.
2.4. Evaluation of Total Flavonoids
The total flavonoid content was determined with rutin as reference compound [19]. Standard rutin solutions were prepared from 0.05 g rutin in EtOH. For the plant extracts’ determination, each one milliliter plant extract (10 mg/ml in 70% ethanol or H2O) was mixed with 1 ml aluminum trichloride (AlCl3) in EtOH (20 g/L) and diluted with EtOH to 25 ml. The absorption at 415 nm was recorded after 40 min at RT. Blank samples were prepared from 1 ml plant extract and one drop of acetic acid, and diluted to 25 ml (EtOH). The absorption of rutin solutions was measured under the same conditions. All determinations were carried out in triplicate. The amount of flavonoids in plant extracts, expressed as rutin equivalents (RE), was calculated by the following formula:
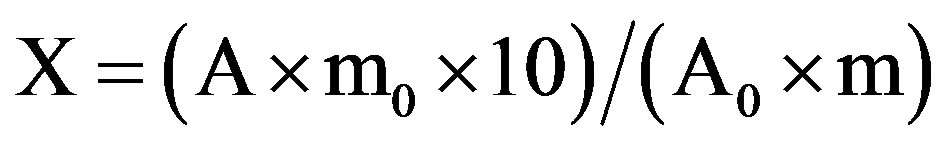
where: X is the flavonoid content, mg/g plant extract in RE; A is the absorption of plant extract solution; A0 is the absorption of standard rutin solution; m is the weight of plant extract (g), and m0 is the weight of rutin in the solution (g).
2.5. Radical Scavenging Properties Assessment
The radical scavenging activities of aqueous and hydro-alcoholic extracts of A. fragrantissima were evaluated using both, 2,2’diphenyl-1-pycril hydrazyl (DPPH) and 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) and (ABTS)-radical scavenging activity as described earlier [20]. All measurements, in triplicate, were performed at 25˚C. Trolox (10−3 mol·L−1) and DPPH (3 × 10−3 mol·L−1) stock solutions (in MeOH) were freshly prepared and stored in dark under argon atmosphere at 4˚C. The Ultraviolet and visible (UV-VIS) spectrometry procedure was carried out by measuring the changes in the value of the DPPH maximum absorbance at 519 nm. In the present study, Trolox equivalent scavenging capacity (TESC) was evaluated instead of the determination of IC50. Using this procedure, the extracts efficacy against DPPH radical was compared to that exhibited for ABTS cation radical. For DPPH, the determinations were performed according to the optimum measuring time settled on the base of system stability 6 min after antioxidant samples additions. All data were expressed as Trolox Equivalents Antioxidant Capacity (TEAC) equivalent to the mass of plant material used in extracts preparation. On the other hand, the evaluation of trolox antioxidant capacity equivalent against ABTS cation radical was performed by generating the ABTS+ subsequent ABTS reaction with potassium persulphate. In this method, fixed wavelength (731 ± 2 nm) was used.
2.6. High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS) Evaluation of the A. fragrantissima Extracts
Polyphenol quantitation in the tested extracts was achieved as described in [21]. The HPLC-DAD-MS measurements were performed using a complete HPLC SHIMADZU system, Nucleosil 100 - 3.5 C18 column, KROMASIL, 100 × 2.1 mm. The system was coupled to a MS detector, LCMS-2010 detector (liquid chromatograph mass spectrometer), equipped with an electrospray ionization (ESI) interface. The HPLC-column was equilibrated for 1 h before injections were started. The mobile phase was a gradient prepared from formic acid in water (pH = 3, solvent A) and formic acid in acetonitrile (pH = 3, solvent B): 0.01 to 20.00 min, 5% to 30% solvent B; 20.00 to 40 min, 30% solvent B; 40.01 to 50.00 min, 30% to 50% solvent B; 50.01 to 52.00 min, 50% to 5% solvent B; 52.01 to 70.00, 5% solvent B. The flow rate was: 0.01 to 5.00 min, 0.1 ml/min; 5.01 to 15 min, 0.2 ml/min; 15.01 to 35 min, 0.1 ml/min; 35.01 to 60 min, 0.2 ml/min; 60 to 70 min, 0.1 ml/min. The mobile phase was sonicated in order to eliminate the dissolved air and then subjected to filtration using a PTFE 0.2 µm membrane. The samples were filtrated before injection using syringe driven filter unit 0.2 µm (Macherey-Nagel). The analyses were performed at 20˚C for the period of 70 min. Then the column was washed over a period of 15 min with mobile phase using the flow rate 0.1 ml/min. After completion of series of analyses, the HPLC system was cleaned with water and MeOH for 1 h. ESI source and negative ionisation mode was used. Nitrogen was used as the nebulising and drying gas. The SCAN (m/z 50 to 800) mode was used for identification of hesperidin and the SIM mode was used when a search for some particular ions should be done.
2.7. Antimicrobial Screening
The antimicrobial activity was evaluated by determining minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) by broth microdilution method [22]. In addition to two fungal species (Candida. albicans ATCC 10231 and C. glabrata ATCC 15126), different Gram positive (Staphylococcus aureus ATCC 3386, clinical strain methicillin resistant Staphylococcus aureus (MRSA) 755, Streptococcus pneumoniae ATCC 49619, Bacillus cereus ATCC 14579 and Enterococcus faecalis ATCC 29212) and Gram negative bacteria (Klebsiella pneumoniae ATCC 1388, Shigella sonnei ATCC 9290, Pseudomonas aeroginosa ATCC 1014, Escherichia coli ATCC 35218 and Salmonella typhimurum ATCC 14028) were used in screening the antimicrobial potency of the A. fragrantissima extracts.
2.7.1. Culture Media and Inoculum Preparation
Frequently subcultured bacteria strains were maintained on nutrient agar (NA) and blood agar (BA) plates. For antimicrobial assay, microbial cultures were freshly grown at 37˚C and they were appropriately diluted in sterile normal saline and adjusted to 0.5 McFarland standards. The yeast strains were grown overnight at 37˚C on Sabouraud dextrose agar plates (Oxoid), and inocula for the assays were prepared by diluting the cultures in 0.85% NaCl solution and adjusted to 0.5 McFarland standard. Protocols followed were in accordance to guidelines of the National Committee for Clinical Laboratory Standards (NCCLS) [23,24].
2.7.2. Antimicrobial Susceptibility Assay
For bacterial cultures, the agar well diffusion method was used by spreading 50 μl of diluted inoculum (105 CFU/ml) of test organism on Muller Hinton agar plates (Oxoid) according to NCCLS guidelines. However, yeast suspensions, were diluted to obtain 104 CFU/ml and they were spread on Sabouraud dextrose agar plates. Wells of 6 mm diameter were punched into the agar medium and filled with 50 μl (100 mg/ml) of plant extract and solvent blanks. The plates were incubated for 18 h at 35˚C. Antimicrobial activity was read by measuring the zone of inhibition. Positive controls were gentamicin (10 µg) (Oxoid) and fluconazole (25 µg) for bacterial strains and yeast, respectively.
2.7.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) by Broth Microdilution Method
Stock solutions (100 mg/ml) were prepared and serially diluted in 96-well plates (Nunc). The final concentrations ranged from 100 to 5 mg/ml when reconstituted with bacterial and yeast suspensions individually. The wells were inoculated with bacterial suspension of 5 × 105 cfu/ml according to NCCLS M7-A5 and with 1 × 105 cfu/ml candida strain according to NCCLS M-27. The microplates were incubated for 24 h at 35˚C in duplicates. One of the 12 columns served as growth control (bacterial suspension or yeast without plant extract); on the other hand, sterile water and 80% methanol were used as negative control. Gentamicin and flucanazole solutions (Sigma) were used as positive controls for bacteria and Candida species, respectively. Readings were obtained by visualizing the lowest concentration without visible growth and was defined as MIC.
For MBC determination, 20 µl from each well from MIC assay plates that showed complete inhibition 100% after 24 h of incubation were sub-cultured on blood agar and incubated at 35˚C for 24 h. MBC was determined as the lowest concentration that showed either no growth or fewer than three colonies to obtain an approximately 99 to 99.5% killing activity. All experiments were carried out in triplicates. Stock solution for the EtOH hydroalcohol extract was prepared using 80% MeOH. Methanol (80%) was used as a control which did not have any effect on the strains used.
2.8. Platelet Aggregation
In vitro antiplatelet activity of the hydro-alcoholic extract of A. fragrantissima was measured by the whole blood Chrono-log 700 lumi-aggregometer using an electrical impedance method as reported recently [25]. ADP and collagen were used as agonists of platelet aggregation and aspirin as positive control.
2.9. Cytotoxicity Assay
The MCF-7 cell line was subjected to cytotoxicity assay. For adherent cells (PLF), 1 × 104 cells were seeded in each well. Colorimetric Cell Titer 96 non-Radioactive Cell Proliferation Assay (Promega, Madison, USA) was used to detect cells proliferation in each well as per manufacturer’s instructions. Briefly, 15 µl of dye solution were added on each well. The cells were incubated at 37˚C and 5% CO2 for 4 h. Then, 100 µl of the solubilization solution were added to solublize formazan precipitate. The absorbance was recorded at 570 nm using a colorimetric plate reader (Sunrise-Basic TECAN, Austria). Cellular proliferation was expressed as a percentage of cell viability of MCF-7 cells relative to untreated controls.
2.10. Acetylcholinesterase (AChE) Inhibition Assay
AChE inhibitory capacity of A. fragrantissima extracts was tested using Thin Layer Chromatography (TLC) assay method [26]. The assay was carried out using a silica gel G60 F250 plate that had been eluted with chloroform:methanol (80:20) and sprayed with naphthyl acetate and Fast Blue B solution mixture. Eserine was used as inhibition standard.
3. RESULTS AND DISCUSSION
3.1. Evaluation of Total Phenolic Compounds and Total Flavonoid Content
Antioxidant and free radical scavenging activities of different Achillea species had been attributed to their large contents of polyphenolic compounds, in particular flavonoids, phenolic acids and tannins [25,27,28]. The evaluation of total phenoland total flavonoid contents in the present study revealed that total phenols content (expressed as mg gallic acid equivalents/g plant extract) was higher for hydro-alcoholic extract (18.94 ± 0.26) compared to aqueous extract (16.63 ± 0.43) (Table 1). Similar observation was made with flavonoid content (mg/g plant extract), in rutin equivalentsand found to be (1.67 ± 0.00) for hydro-alcoholic extract and (0.95 ± 0.02) for the aqueous extract (Table 1).
3.2. Antioxidant and Radical Scavenging Activities
The radical scavenging activities of both extracts of A. fragrantissima were evaluated using the methods of DPPH and ABTS radical scavenging activity assays. The scavenging capacity is expressed in Trolox equivalent antioxidant capacity, (TEAC).
Both extracts exhibited DPPH and ABTS radical scavenging activity. Again, the hydro-alcoholic extract exceeded the potency of the aqueous extract in both methods (Table 2).
In the tested extracts the value of TEAC against ABTS+ are higher with respect to TEAC obtained against DPPH. DPPH and ABTS are free radicals that react on the base of a combined mechanism, equally as Hydrogen Atom Transfer (HAT) and Electron Transfer (ET).
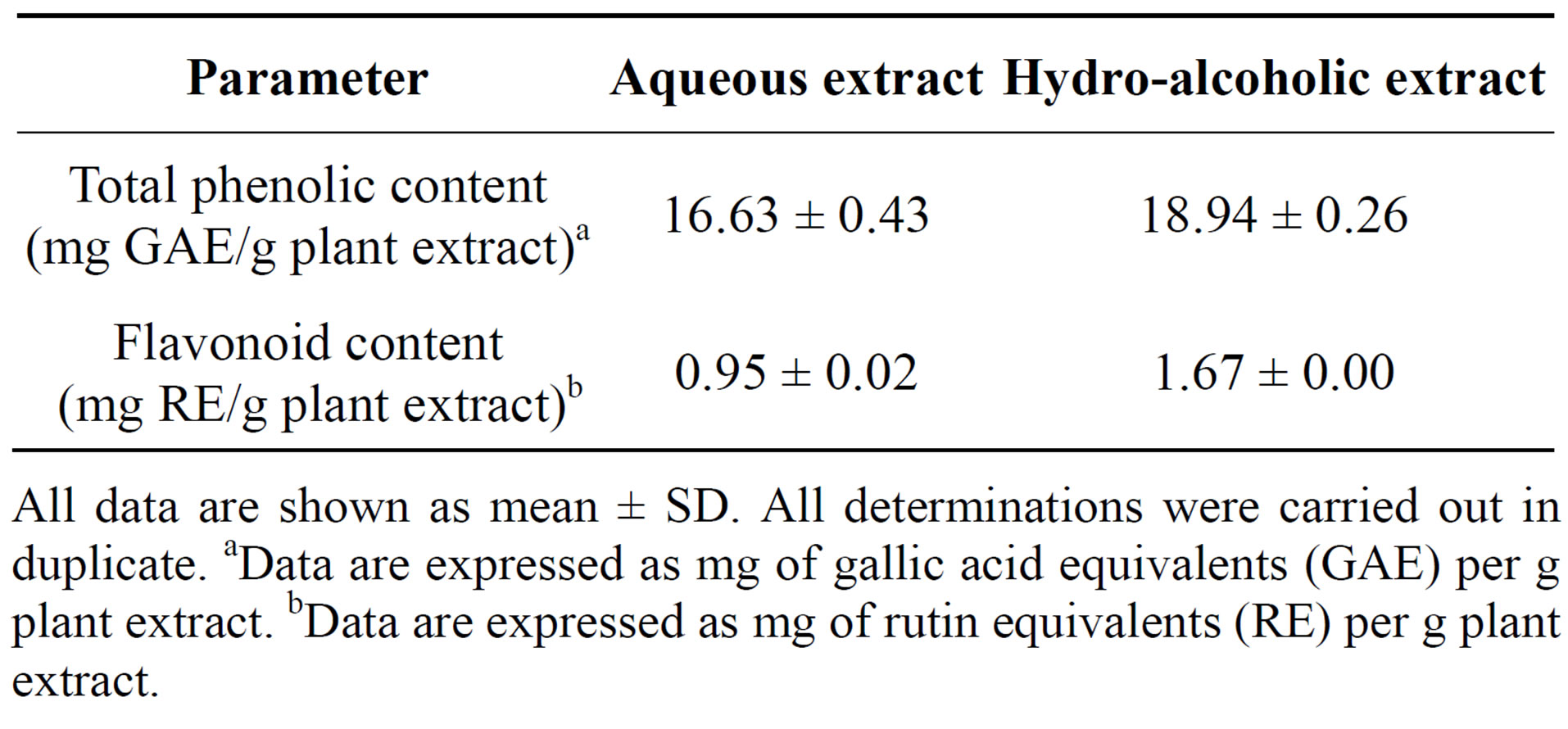
Table 1. Total phenolic content and total flavonoids of aqueous and hydro-alcoholic extracts of A. fragrantissima.
All data are shown as mean ± SD. All determinations were carried out in duplicate. aData are expressed as mg of gallic acid equivalents (GAE) per g plant extract. bData are expressed as mg of rutin equivalents (RE) per g plant extract.
DPPH reacts only with flavonoids having an OH group at ring B or with aromatic acids with single OH group. ABTS does not discriminate between OH phenolics providing a response related with total groups able to quench a radical reaction. Hence, DPPH scavenging highly depends on the degree of electrons delocalization [29].
In a study of ethanolic extract of A. fragrantissima for its anti-inflammatory effects on lipopolysaccharide (LPS)- activated primary cultures of brain microglial cells, the extract components acted as efficient intracellular hydroperoxyl radical scavengers [15]. Our results showed an agreement with a previous study of antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes where both aqueous and methanolic extracts of A. fragrantissima exhibited antioxidant capacity that was associated with the phenolic compounds of the extracts [17].
3.3. High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS) of the Extracts
Seven compounds were identified in the hydro-alcoholic extract compared to four compounds identified in the aqueous extract (Table 3, Figures 1 and 2). Querce-
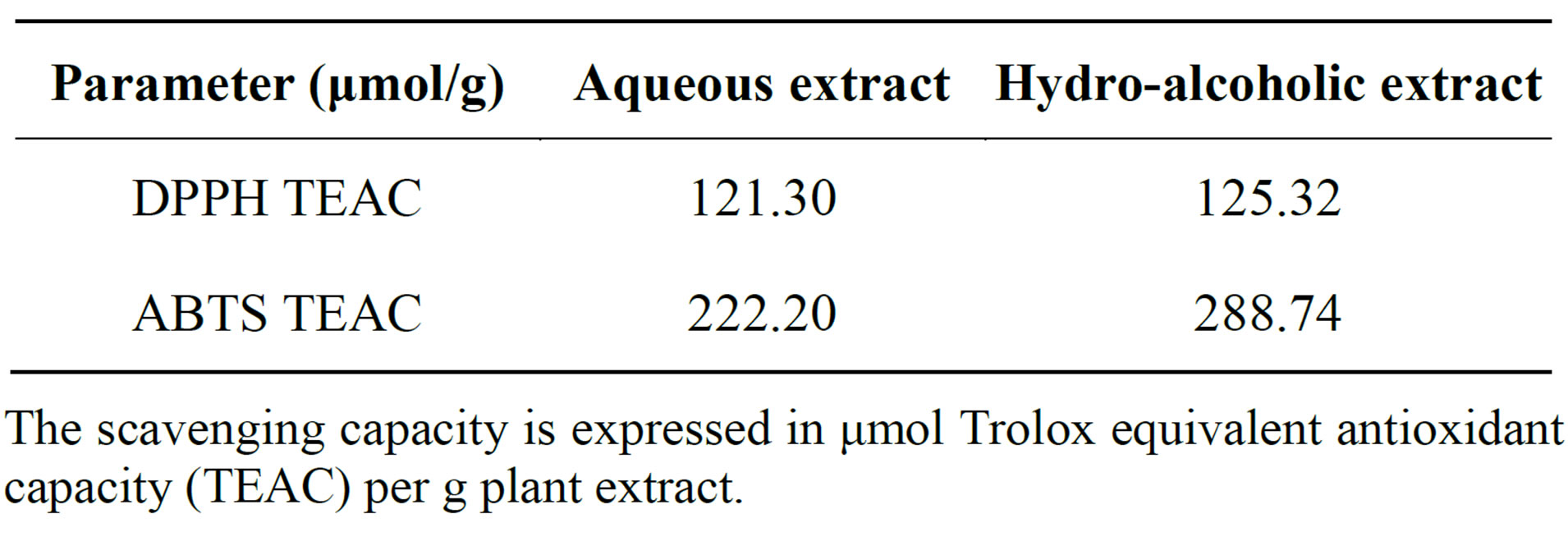
Table 2. Antioxidant activity of aqueous and hydro-alcoholic extracts of A. fragrantissima.
The scavenging capacity is expressed in μmol Trolox equivalent antioxidant capacity (TEAC) per g plant extract.

Table 3. HPLC-MS pattern and polyphenolic content of aqueous and hydro-alcoholic extracts of A. fragrantissima.
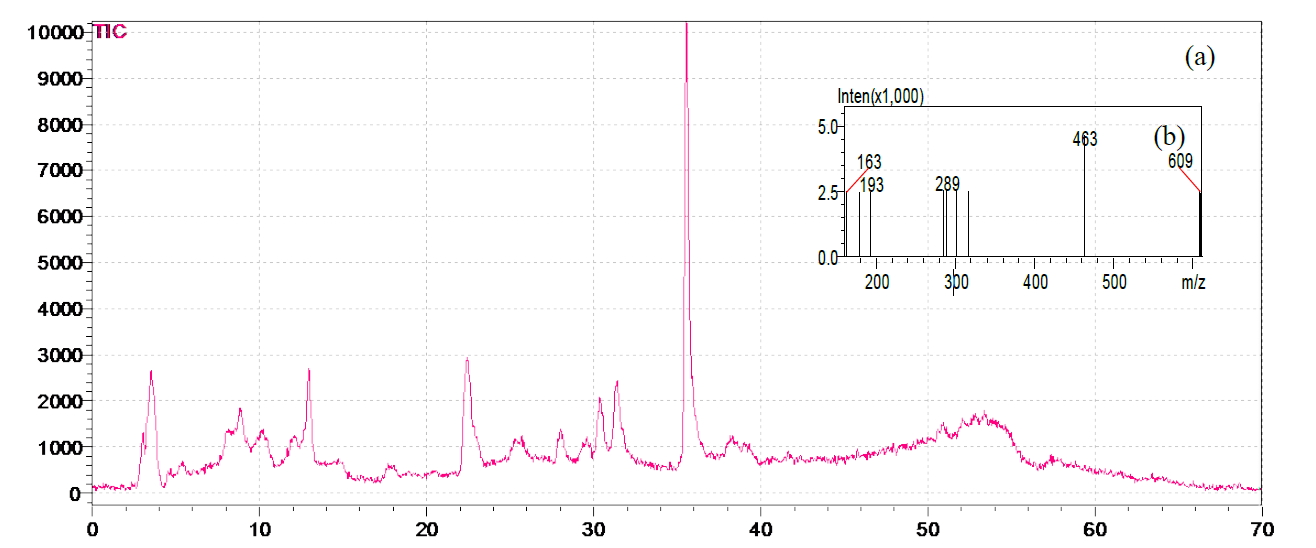
Figure 1. (a) HPLC-MS chromatogram of A. fragrantissima aqueous extract; (b) Mass spectra total ion current (TIC) of the corresponding sample.
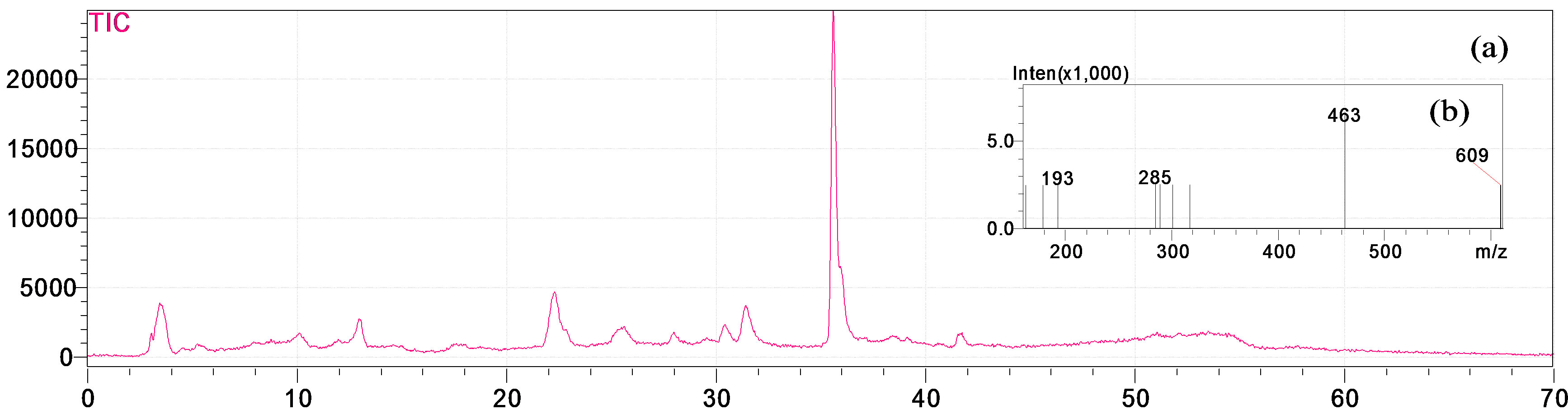
Figure 2. (a) HPLC-MS chromatogram of A. fragrantissima hydro-alcoholic extract; (b) Mass spectra total ion current (TIC) of the corresponding sample.
tin 3-β-D-glucoside was dominant in both, hydro-alcoholic (631.46 μg/g) and aqueous (345.6 μg/g) extracts, followed by ferulic acid (33.52 μg/g) and (21.36 μg/g) for the hydro-alcoholic and aqueous extracts, respectively. El-Shazly et al. (2004) determined the chemical composition of the essential oil and n-hexane-ether extract of A. fragrantissima, growing in Sinai desert, using GLC and GLC-mass spectrometry analysis [30]. To the best of our knowledge, this is the first investigation of the chemical composition of aqueous and hydro-alcoholic extracts of A. fragrantissima.
3.4. Antimicrobial Activity
Antimicrobial activities of A. fragrantissima aqueous and hydro-alcoholic extracts were evaluated by the agar well diffusion method. MIC and MBC were determined by microdilution method against well known pathogenic gram positive and gram negative bacteria and yeast. All Gram positive bacteria in the agar well diffusion test for aqueous extract (Table 4). However, the tested extracts failed to show any significant activity for gram negative bacteria and for both strains of Candida [31].
Results also showed that the hydro-alcoholic extract exhibited bactericidal activity against S. pneumoniae and B. cereus rather than inhibitory effect. Previous studies showed that essential oil of A. fragrantissima exerted a bactericidal effect on several gram positive and gram negative bacterial strains, as well as on C. albicans [30,32].
3.5. Platelet Aggregation
In Jordanian folk medicine, Achillea species are used as a haemostatic to stop bleeding [9]. In support of the traditional use of the genus Achillea in the Jordanian folk medicine A. bieberstienii has been reported to enhance platelet aggregation while similar reports do not exist for A. fragrantissima based on the information available to us in the literature [25]. The hydro-alcoholic extract of A. fragrantissima was tested for its antiplatelet activity on human whole blood in vitro, without any detectable effect on platelet aggregation at concentrations of (25, 50, 100, and 200 μg/ml).
3.6. Cytotoxicity Effect
The anti-proliferative potential of extracts from A. fragrantissima is not fully studied yet. A recent study reported cytotoxic effect of an aqueous extract of A. fragrantissima against HepG2 human hepatocellular carcinoma cells [33]. The MTT assay was used as a relative measure of cell viability to study the anti-proliferative activity of the aqueous and hydro-alcoholic A. fragrantissima extracts against the MCF-7 cells. At concentrations up to 200 μg/ml, extracts did not possess cytotoxic activity.
3.7. AChE Inhibition Assay
The neuroinflammatory process plays a central role in

Table 4. Antimicrobial activity of ethanol and aqueous extracts of A. fragrantissima.
*Antimicrobial activity against Candida results are not shown in the table. **R: Resistant.
the initiation and progression of neurodegenerative diseases such as Parkinson’s and Alzheimer’s diseases (AD). The latter is due to the reduction in levels of the neurotransmitter acetylcholine, in the brains of the elderly as the disease progresses, resulting in loss of cognitive ability [34]. A variety of plants has been reported to show AChE inhibitory activity and so may be relevant to the treatment of neurodegenerative disorders such as AD [35]. Using TLC assay method, neither aqueous, nor hydroalcoholic extracts showed AChE inhibition. Nonetheless, A. fragrantissima has been reported to possess antioxidant and anti-neuroinflammatory activities that could be beneficial in preventing/treating neurodegenerative diseases in which neuroinflammation is part of the pathophysiology [15].
4. CONCLUSION
The present investigation using the aqueous and hydro-alcoholic extracts of A. fragrantissima supported the traditional use of this plant in the Jordanian folk medicine as an antimicrobial active representative of the genus Achillea, and provided the exact identification of the species recommended. This is necessary since all Achillea species were referred to locally in Jordan as “Qaysoum” and recommended for a big variety of diseases despite differences they exhibited in scientific evaluation [36]. A. fragrantissima extracts should be further evaluated as for their potential use in preventing/treating diseases in which oxidative stress is a part of the pathophysiology.
ACKNOWLEDGEMENTS
This work was supported by a grant of the Deanship of Academic Research, The University of Jordan. The authors would like to thank Mr. Ismail Abaza and Mrs. Amal Abualraghib for technical help.