1. Introduction
Wilt symptoms caused by the soil-borne saprophyte Verticillium dahliae account for severe yield losses in numerous culture and cash crops and raise the risk of fruit and vegetable production. Albeit strawberries are a cash crop of intermediate economic importance in Germany, the market for regional fruit is not saturated and strawberry cultivation has expanded in Germany over the last few years to coverage of 14,000 ha in 2011. Verticillium dahliae is the most important pathogen in strawberry production besides Phytophtora cactorum [1], and is explicitly quoted as a major risk that stands against an increase in production by horticultural services, experimental research and growers. Preventive measures to avoid plant infection are expected to significantly contribute to sustaining strawberry cultivation as a culture crop in traditional fruit producing regions such as the region of Brandenburg that surrounds Berlin, Germany.
The incidence of the fungus in soils is of irregular intensity and can be unevenly distributed within a plot [2] while plants show a varied tolerance towards fungal colonization [3]. Interrelated with the occurrence of asymptomatic host plants [4] this entails the intricacies of a meaningful assessment of risk in fruit production. Furthermore, growers encounter difficulties in avoiding or restoring infested sites, partly due to the long term persistence of microsclerotia (MS) in the field and partly due to the limited availability in cropland within the individual farm or even within a region [5]. As a result of the phase out of biocides chemical biocide usage (EU regulation No. 528/2012) and an absence of alternative control measures or management strategies [6], a practicable method of wilt regulation is strongly demanded by farmers in Germany, and is thus subject of this study.
An extensive review on Verticillium wilt disease in olive describes a multitude of factors influencing disease onset, progress and severity, including agronomical factors such as irrigation, fertilization and soil management as well as environmental factors such as temperature, edaphic features and biotic interactions [7]. The authors conclude that symptoms depend mainly on cultivar susceptibility, inoculum potential in the soil and environmental conditions. The latter strongly correlates with cultural practices employed in commercial agriculture [8]. From a farmer’s point of view the synthesis of current knowledge implies an estimation of soil quality as well as plant quality and a selection of plot management measures to be applied before, during or after planting in order to influence environmental conditions in favor of wilt regulation. All practical approaches are addressed in scientific research.
For many years, analysis of Verticillium infestation in soils based on wet-sieving methods as a risk prediction method provided useful to strawberry growers in spite of evident limitations such as a dependence on weather, soil type and preceding crops [2,9]. Progress was achieved with the development of qPCR methods to detect Verticillium microsclerotia (MS) in soils [10]. This technology is currently being optimized for in vivo detection in plants [11].
Incidence and severity of Verticillium wilt are associated with crop history, i.e. the succession order of susceptible and unsusceptible crops grown on site. Although this is found unreliable for wilt risk assessment [2], it has led to numerous studies of bio-fumigation and crop rotation effects in order to reduce inoculum potential, e.g. [12], albeit with limited overall effect in practice [5]. Other management practices involve manipulation of the soil temperature, handling of plant residues or reducing nitrogen fertilization [13,14].
Plant resistance is addressed on a regional basis by regularly testing cultivar susceptibility and resistance in experimental research involving bioassays, e.g. [15]. However, comparison of results between regions shows heterogeneities and thus does not lead to an overall approach. Corresponding to the understanding that wilt disease occurs more frequently in vegetatively propagated crops [2], further improvements of micropropagated plants were tested by inoculating with different species of arbuscular mycorrhizal fungi, but showed no definitive growth trends [16].
Biological control agents (BCAs) can manipulate the plant rhizosphere by inoculation of plant parts with specific microbes before or after planting by utilizing either antagonistic effects or vitality boosting properties, e. g. due to improved accessibility of nutrients. Studies were conducted with various species, e.g. Pseudomona spp. [17], rhizobacteria isolates [18] or soil-associated fungi [19]. A minor subset of researchers in the field of BCAs focuses antagonistic effects of Verticillium subtypes for Verticillium control. Tyvaert reports results from an interaction of Verticillium longisporum with Verticillium Vt305 isolates used as a BCA in cauliflower plants [20]. Host specificity of Verticillium isolates and varying effectiveness in pathogenicity on strawberries are wellknown [21,22].
Although the majority of the above mentioned studies incorporate field trials, they remain isolated applications only indicating the potential for a holistic strategy that can be applied in the different micro-environments of genuine commercial practice. An upscale or roll-out of methods to meet practice conditions has not taken place to the extent that growers can incorporate scientific progress on wilt management when installing a new plantation, and the question how to keep stock in spite of wilt disease remains unresolved. The need for a validation of methods for wilt management is increasingly being articulated by German farmers. Therefore, the objective of this study was to test the potential use of an innovative management measure to regulate wilt caused by Verticillium under conditions of commercial production.
The proposed measure is based on findings that relate wilt incidence to changes in plant microbial population structures and the observation that wilted plants are colonized by a different number and composition of subtypes with different pathogenicity compared to healthy plants. The BCA (EVI) consists of three non-pathogenic genotypes of Verticillium dahliae shown to successfully control wilt symptoms in strawberry plants in different climate regions [23-26].
Primarily, the focus of this study lies on the technical applicability of inoculation with conidiophores in the common work routine of planting to demonstrate the proof-of-concept independently of field localization, environmental conditions and field management. Monitoring of plant vitality is conducted to reveal plant reactions in dependence of the interaction between soil, plant and the BCA in order to show the potential of the concept by using a parameter directly usable by the strawberry growers for economic analysis.
2. Methods
2.1. Experimental Sites
Two production sites (A, B) that were subject to similar conditions in commercial practice were chosen for experimental testing in Brandenburg, Germany, with a linear distance of 114.8 km (38.9 km west and 79.9 km south-east of Berlin) (Figure 1). Both sites have a history of strawberry production and were characterized as highly infested with Verticillium by the growers. Field inspection conducted in 2011 showed detectable wilt infection on old growth. Spot samples analyzed for Verticillium dahliae confirmed similarity in terms of infestation with microsclerotia for both locations (15 - 20 microsclerotia per gram soil).
Plot A was loamy sand (10% silt, >80% sand) (Soil information system Brandenburg LBGR), located at latitude 52.45˚, longitude 12.80˚, with an elevation 29 - 35

Figure 1. Location of production sites used for field test.
m and a precipitation rate of 592 mm with an average temperature of 8.7˚ (30 years mean). Plot B was loamy sand (silt 10%, sand >85%), located at latitude 52.28˚, longitude 14.75˚, with an elevation of 58 - 61 m and a precipitation rate of 443 mm with an average temperature of 8.5˚.
For each site, two plots were set up in direct vicinity in 2011 (A2011, B2011) and 2012 (A2012, B2012), thus allowing to test different micro-environments per location.
2.2. Molecular Verticillium Detection
Molecular detection of soil-borne Verticillium was performed once in 2011 in order to confirm personal communication with the growers by using a Verticilliumspecific polymerase chain reaction (PCR) assay with primers allowing accurate detection of Verticillium dahliae including lineages of A1/D2 and A1/D3 Verticillium longisporum [27]. Soil DNA was extracted with Nucleospin-Soil DNA Extraction Kit (Macherey-Nagel GmbH, Dueren, Germany) according to protocol. Quantification of Verticillium was achieved using a 5x hot firepol Evagreen qPCR mix + ROX (Solis Biodyne, Tartu, Estonia) (AB 7500 fast, Life Technologies Corp., Carlsbad, CA, USA) with two independent calibration curves derived from Verticillium dahliae microsclerotia. PCR amplification was performed with an initial denaturation at 95˚C for 10 min, followed by 40 cycles of 0:15 at 95˚C. PCR was carried out in 20-μl reactions with 5 pmol of each primer: Forward primer VDP1 (5’-TCTACTCATAAC CCTTTGTGAACCA-3’), reverse primer VDP2 (5’-ACT CCGATGCGAGCTGTAAC-3’).
2.3. Test Design
The test design was developed under consideration of irregular dispersal of fungi in soils and erratic intensity of soil infestation within fields. Taking the individual planting techniques used by the growers into account, we designed experimental rows and thus refrained from the traditional randomized block design. In consequence, plants with EVI treatment were located in direct neighborhood of control plants. The main strategy was to have a neighborhood comparison with EVI treated plants and the control and to observe either constant or differential effects of plant reaction to EVI by comparing pairwise among each variant. Control plants were expected to be susceptible in contrast to EVI treated plants. Testing was conducted in two successive years from March till September 2011 and 2012.
Plot A: Planting was conducted by a four-hole-plantlogger machine fed manually (four rows simultaneously) providing an in-between plant distance of 30 cm. Planting was conducted on June 3rd 2011 (A2011) and June 14th 2012 (A2012). Test design for A2011 comprised 16 rows with four double-rows with EVI treatment next to control plants, each double-row alternating with two intermediate rows. Each row contained approx. 420 plants. Test design for A2012 comprised four rows with two double-rows. First double row alternating EVI and control, the second double-row alternating EVI + root cut and control + root cut. Each row contained approx. 220 plants.
Plot B: Planting was conducted by hand following a three-furrow hole puncher providing an in-between plant distance of 33 cm between plants (three rows simultaneously). Planting was conducted on May 19th 2011 (B2011) and May 25th 2012 (B2012). Test design for B2011 comprised four rows with two double-rows alternating EVI and control. Each row contained approx. 400 plants. Test design for B2012 comprised 3 rows alternating control, EVI, control + root cut, thus flanking one row with EVI treatment with one row of control and one row of control + root cut on either side. Each row contained approx. 400 plants.
Plot C: For a gain in clarity, we set up a third plot on the institutes premises on virgin soil with no known history of cultivation and no indication Verticillium infestation in 2011.
2.4. Plant Material
Treatments were performed on strawberry plants (Fragaria × ananassa [Duchense] Decaisne & Naudin [family: Rosaceae]) using “Elsanta” which was prioritized by regional growers as a preferred cultivar in fruit production due to its favorable characteristics for trade (personal communication). The cultivar was found highly susceptible to Verticillium in this region, and was thus used for testing in correspondence with findings that aggressiveness in isolates is most significantly expressed in the highly susceptible genotype cultivars [28]. Plant material for field testing was retained from the batch prepared for planting for plots A and B. Plant material a and b was available in tested quality A++ (A2011, B2011) and A+ (A2012, B2012). For Plot C own plant material was used (c), available in a tested quality A+++, as well as plant material in tested quality A++ taken from leftovers of plant material from Plot B, source b.
2.5. EVI Treatment
Treatment of plants was conducted after removal from cold storage and immediately before planting to reproduce actual work flow. We inoculated the plants under controlled conditions, while preparation of beds, planting, irrigation and further treatment were carried out by the growers according to individual techniques.
EVI contained a mixture of non-pathogenic strains of Verticillium dahliae prepared in cooperation with ABiTEP GmbH, Berlin, Germany for investigating technological upscale in fermentation. The soluble compound included an equal proportion of spores from each of the three isolates of Verticillium dahliae resulting in a final concentration of more than 106 conidia spores/ml per strain.
Plant roots were immersed in an aqueous suspension with a concentration of 1 × 105 conidia spores/ml and 5 × 105 conidia spores/ml corresponding to findings that inoculation with a spore suspension of about 106 conidia per ml using a root-dip technique was efficient for affecting plant reaction to Verticillium dahliae isolates as well as for precisely distinguishing tolerant from susceptible cultivars [21,22,29]. Inoculation by root dip was tested for 30 min and 40 min in solution with best results at 30 min. Inoculation was also tested for plant material with clipped roots (root cut) and for plant material without infringement.
2.6. Inoculation Effectiveness
Regular, clipped and inoculated plant material was retrieved from the batch and subsequently stored at 4˚C for microbiological analysis. Adhering conidiophores on the root surface were determined by shaking the roots in 0.9% solution of sodium pyrophosphate under addition of sterile 2 - 5 mm quartz gravel for 15 min. This suspension was plated in different stages of solution to identify the number of Verticillium colonies. The number of conidiophores inside the roots was determined by plating the rinsed roots on culture medium in 0.5 cm pieces. Analysis was conducted for the different plant materials. Plant material c had an average root weight of 12.7 g; plant material a and b had an average root weight of 7.4 g.
2.7. Plant Vitality and Disease Assessment
Plant vitality was rated by visual monitoring performed 3 - 3.5 months after planting in each year and for each plot (A2011: August 22nd 2011, A2012: August 23rd 2012, B2011: August 5th 2011, B2012: September 12th 2012). Plant vigor was scored on a scale from 0 (dead wilted plant) to 9 (healthy with no wilt symptoms). Plants with a score >7 were considered vital, according to the growers rating of plants for commercial use.
Development of wilt was assessed and recorded for all plants treated with EVI and the control plants in the test design. Plants were rated pairwise across rows. Intermediate rows between test rows were observed for peculiarities but not rated. Visual rating was later extended to record symptoms for other pathogenic diseases (Nematodes, Alternaria sp., Diplocarpon earliana, Mycosphaerella fragaria, Phytophtera sp.).
2.8. Re-Isolation
Plant samples were cut into 1 - 2 cm pieces. For surface sterilization pieces were initially treated by stirring in Iso-propanol followed by distilled water twice and subsequently with hydrogen peroxide. Further the samples were dried for few seconds on a sterile filter paper and treated again with water inside a vacuum chamber. The samples obtained from vacuum glass filter were dried on sterile filter paper and were divided into four pieces. These small pieces were transferred carefully on to potato dextrose agar media. The plates were incubated at 25˚C until the colonies are formed. Verticillium dahliae and Alternaria sp. were identified by phenotypical features in microscope analysis.
3. Results
Technical applicability of inoculation with conidiophores in the common work routine of planting was found feasible. Since root dipping is a common measure to be undertaken before planting, application of the measure in the field allowed a fluent integration into the work flow during planting.
Inoculation effectiveness was given, whereas plant material with root cut showed no differences in colonization of the fungus compared to plants with roots kept intact. Plant roots were colonized after 30 min of root dip; however the fungus could not be retrieved from petioles in both cases.
An inoculum concentration of 1 × 105 conidia spores/ ml was found to be sufficient. Plant material showed adherence rates of 2.1 × 105 (source a) and 1.1 × 105 conidiophores per root (source b) on the root surface. Doubling the inoculum concentration to 5 × 105 showed no increase in colonization for both sources of plant material. A saturated concentration was observed at 1.5 × 104 conidiophores per gram root fresh weight.
3.1. EVI Effect on Plant Health
Demonstration of the proof-of-concept in different spatial settings independently of environmental and technical variances and crop treatment was not successful in light of economic viability. Overall plant health could not be improved with the treatment and the number of dead and wilted plants in the EVI treated rows added up to approx. 50% - 60% for both locations and both years. Concluding from the number of dead plants in the EVI rows compared to the control the growers stated that complementing soil parameters with high quality plant material was experienced more stable and that treatment with EVI was no option for future use.
Plants treated with EVI showed 5% - 30% dead plants compared to 1% - 5% dead plants in the control on both Plot A and B (Tables 1 and 2). Vitality rating added up to the same overall result for both plots, but the pattern of dead and wilted plants in 2011 did not correspond with the pattern found in 2012. Vitality rating also showed that EVI treatment did not have homogeneous effect within one plot as some areas were free of dead plants.
A2011 showed an average vitality of 4.5 after EVI treatment due to the high number of dead plants of over 30%. Average vitality without consideration of dead plants slightly decreased from 7 to 6.5.
A2012 showed an average vitality of 7 after EVI treatment. The effect from 2011 could not be repeated in 2012. In 2012, we found a strong correspondence of vitality with the root cut plants. Average vitality excluding dead plants however showed no differences between EVI treated plants, control or control + root cut.
B2011 showed a decrease in vitality along a gradient crossing the rows (7.5/7.6/7.0/6.7). Death rate increased running counter to the gradient (1.1/1.1/3.6/5.3) and leading to an overall decrease in average plant vitality from 7 to 6.5. Average vitality excluding dead plants from the analysis, showed a milder effect of decreasing vitality from 7 to 6.
B2012 showed a very high portion of dead plants adding up to more than 30%, thus showing a similar effect to A2011. However, the effect of root cut did not show the same impact compared to A2012.
Altogether, vitality rating showed an atypical course of disease not common for Verticillium wilt such as rapid wilting. Root cut showed a slight dampening in plant vitality in the field trials but the effect was not reproducible, suggesting an influence of soil microbes other than Verticillium to colonize the plants.
Rating conducted to assess the indication of other pathogenic diseases showed considerable increase of pathogens of other type and culture. Results showed 30% - 40% latent infection rate of vital control plants caused by Phytophtora in both years. Strongly wilted and dead plants (rating grades 0 - 3) showed an infection rate of Phytophtora at 80% - 100% (rated by red colored tissue at section of petiole). In the EVI treated plants, the ratio of dead plants caused by Phytophtora infection was up to 30% compared to 1 - 5 in the control. The potential is more or less evident dependent on location and year.
3.2. Adverse Effects and Plant-Soil Interaction
For every pair of plants according to the sequence of matches along the rows, the reaction of plants towards EVI treatment (indicated by the vitality rating on a scale of 0 - 9) was quantified by calculating the difference between vitality of the EVI treated plant and vitality of the neighboring control, in this case a priori excluding dead plants to avoid a bias from other pathogens.
Pairs that show no difference in vitality were marked as neutral. Pairs that showed a positive vitality of EVI compared with control were marked as positive. Correspondingly, pairs that showed a negative vitality of EVI compared to control were marked as negative.
In the following, data is shown for 2011and 2012. The positive effects showed a consistent boost of vitality of 2 - 3 grades in rating, while the negative effects also showed certain conformity of reduced vitality of 2 - 5 grades in rating (Tables 3 and 4). The positive effect was shown in approx. 20% of pairs, thus referring to an effect in inoculated plants.
An effect of plant material was noticeable in the visible curb of vitality. Visual inspection of the sites had also shown the before mentioned gradient in wilt infection crossing rows.
Plot C conducted in parallel on the premises of the institute was now drawn on, to analyze whether indications of influence either from observed differences in the micro-environment determined by the soil or from observed disparities determined by different quality of plant material could be substantiated (Table 5).
Results showed that independent of plant material a slightly positive effect of EVI treatment can be proved based on the rating of plants pairwise across rows in Plot C, which was not pre-infested with Verticillium. Analysis also showed a strong effect resulting from plant mate-

Table 3. Plant reaction rating (A).

Table 4. Plant reaction rating (Plot B).
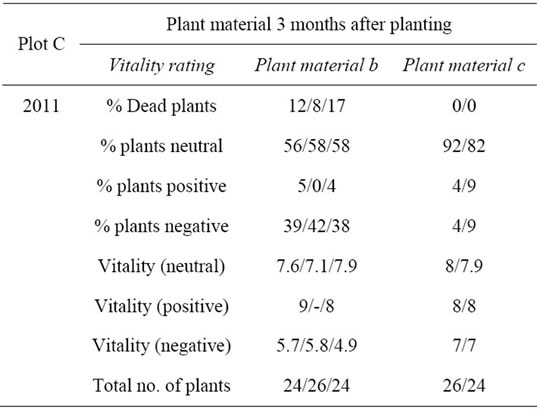
Table 5. Plant reaction rating (Plot C).
rial a which had a strong negative impact on the vitality of EVI treated plants (reduced vitality to 5.5) and in the extent of plants affected (40% below 7 and 12% dead). Rating from EVI treated plants from plant material a in Plot C was conform with A2011 (60% below 7% and 33% dead). Plant material c showed none of these effects.
A more detailed analysis of the additional rating conducted to assess the incidence of other pathogenic diseases showed not only an increase of microbes on the whole but also significant shift in microbe populations in the EVI treated plants: the microbe population was found to be of a different composition compared to control.
Pairs of plants were retrieved from the field at the date of vitality rating. Pairs were selected individually to analyze neutral, positive and negative influence of treatment in EVI treated plants, each against the neighboring control plant. Petioles from control plants showed 59% no growth, EVI treatment halved this effect to 35%, showing that colonization on the whole had doubled. Besides an impact on quantity of microorganisms, the specter of groups was changed and more diverse (Table 6).
4. Discussion
Vitality rating led to results contradicting previous experiments in laboratory, greenhouse and field conducted in 2005 [24,25]. Consequently, evidence for a proof-ofconcept independently from spatial settings is not given. The application of a BCA in form of inoculation with apathogenic Verticillium strains is found to show a different trend neither expected nor estimated when applied under commercial growing conditions out of the laboratory.
Nevertheless, the positive effect of the BCA can be shown in the detailed analysis of pairwise plant rating. The developed test design in rows was found extremely useful for this detailed plant-to-plant comparison along local unknown complex differences in interactions.

Table 6. Ratio of re-isolates in plants with EVI treatment + root cut (r.c.) and control (C) as seen from petioles (A-L phenotypical characterization of fungal groups).
4.1. Application of the EVI Control Agent
Based on the outcome that regulation of wilt was successful, but enhanced the pathogen background to a complex course of diseases, further technological development of EVI application is currently found to be impaired by side effects and quality of plant material.
EVI is found to disrupt the given plant-soil interaction (as in control) and lead to a strong interference of other pathogens involving for instance Alternaria sp. and Phytophtora sp. The found change in quantity and diversity of microbial composition in the plant triggers a sequence of diseases leading to plant loss. Adverse effects differ from location to location and from year to year resulting in different proportions of dead plants. Successful field application of EVI will depend on further research and monitoring of interdependencies in soil-borne diseases in plants and plot in order to address the complex interaction of such side effects in wilt regulation.
Phenomena in the field influenced by natural environment factors such as temperature, humidity, microbiological interactions resulting in changes of pathogen concentration in the plant surrounding can be partially reduced by triennial seasonal observations that make evaluation more reliable but also more costly and timeconsuming [29]. However, plant-soil interaction is a black-box when it comes to application of regulation measures under commercial conditions. Key factors for such side effects are found in the pre-infestation of plant material or soil by other pathogens. For an application of EVI we were able to show the significance of determining interactions with other pathogens because of results that show an influence of inoculation not only on the plant system but also on the microbial system.
Other unpredictable results were found, e.g. in an influence of wilt development by nematode infestations in soil [2] or in microbiological interactions resulting in changes of pathogen concentration in plant surroundings [29]. Chemical fumigants can also strongly influence the microbial interactions by being partly selective to microorganisms that could increase pathogen re-colonization [30]. This effect has been studied in the field applying the fumigants to strawberry fields under commercial agricultural practice [31].
4.2. Role of Plant Material
Results strongly suggest that the course of disease was triggered by the BCA application but was in effect caused by other pathogens in the soil. In our case, the latent infection in a large part of control plants caused by Phytophtora (30% - 40%) was laid open by the treatment with the BCA. Certified plant material of very high quality did not show the same side effects but showed results as we had expected from our previous studies.
The homogeneity and quality of plant material is one significant factor, and therefore one condition, for a reliable application of BCAs under commercial conditions. Additionally, the level of quality in strawberry plant material is also economically relevant regarding the co-import of pathogens by importing plant material [32]. The relevance of infection spread by nursery plants was described for olives already in 1993 [33]. For an application of BCAs under commercial conditions we therefore suggest to use certified plant material tested not only for Verticillium but also for other pathogens such as Phytophtora and Alternaria.
4.3. Role of Pre-Infested Soils
Under conditions of pre-infested soils on middle to high level as in Plot A and B (15 MS/g), re-isolation showed Verticillium infestation of 20% in the control plants. EVI treated plants likewise show a positive effect of approx. 20%, no effect on Verticillium free soils as in Plot C. On not pre-infested soils EVI had no impact. This was shown independently of the plant material.
5. Conclusions
The concept of apathogenic Verticillium application was found to have specific positive effects on the occurence of Verticillium wilt under commercial conditions. However, the effect is overlaid by the interaction of the BCA with other pathogens in the soil or in plants induced by the EVI treatment. Any manipulation of plants such as root cut can increase the side effects extra.
Application of a BCA in strawberry plantations as shown here needs additional specific requirements, such as certified plant material or soil analysis for other pathogens besides Verticillium to obtain healthy plants.
6. Acknowledgements
The authors gratefully acknowledge the funding granted by the Landwirtschaftliche Rentenbank, Germany (FKZ 28LR40-007) for financing a technology transfer project that was basis for this study. We owe valuable insights to our transdisciplinary research team, namely Melanie Mechler, Ilona Bartelt, Chandan Chiniga Kemparaju and Dr. Helmut Junge (ABiTEP). We also thank the strawberry growers in Brandenburg who cooperated with us.
NOTES