1. INTRODUCTION
Blue swimming crab, Portunus pelagicus, is distributed throughout the coastal waters of tropical regions of the Indo-west Pacific [1,2] where it supports a significant fishing industry [3] around the world. P. pelagicus is a commercially important species in Malaysia, for local consumption or for culture which is caught from the sea [4]. Recently, the crab fishery and culture operations are expected to continue to grow in the future [5]. However, problems associated with the use of live foods in aquaculture hatcheries have become a major bottleneck for expansion of the crab farming industry [6]. Improvement of current hatchery protocols is therefore necessary.
Although, both combination diet of Brachionus sp. and Artemia sp. was successfully used for P. pelagicus larvae [7,8], a different live food organism or algae gained popularity because the fact that a single or mixed organism or algae could not fulfill all larval-stage diet requirement. Studies showed that other than rotifer and Artemia nauplii, green plytoplankton and mixed diatom were also used to feed Portunidae crab larvae [9-12]. Commercial artificial encapsulated larvae feed was also used to feed Portunidae crab larvae [13,14]. Instant frozen Nannochloropsis spp. used in the present study was produced by Reed Mariculture Inc., USA. These instant algae are 1 - 2 μm in cell size, 68 billion per ml in cell count and 18% in dry weight. The algae content analysis is 52% protein, 12% carbohydrate, 28% lipid and 37% EPA of lipid. Encapsulated Spirulina spp. used in the present study is produced by Seastar International Inc., Salt Lake City, USA. These filamentous fine dark blue-green algae were washed through fine 20 μm mesh plankton net and mixed with water prior to feeding. The algae content analysis is 68% crude protein, 15% carbohydrate, 6% crude fat, 9% ash (minerals), 2% crude fibre and 2% moisture. The artificial encapsulated shrimp larvae feed content analysis is 56% crude protein, 9% lipid, 1.9% crude fibre and 8% moisture. According to Lavens and Sorgeloos [15], artificial diets offer a reduction in problems and risks involved in the production of the live food can meet better the food size requirements of the predator and can be more effective energetically.
This study aimed at identifying appropriate food and feeding regimes of P. pelagicus for maximizing larval survival and growth by evaluating the efficacy of frozen food organism (Nannochloropsis oculata and encapsulated Spirulina) and an artificial diet in the larval rearing of P. pelagicus.
2. MATERIALS AND METHODS
2.1. Experimental Design
Newly one day hatched larvae were used in this experiment. Six treatments were conducted through the study period with three replicates for each treatment for different diets with live food (rotifer and Artemia nauplii) as continues feed. These six types of treatments are with and without instant Nannochloropsis oculata, with and without instant encapsulated Spirulina and with and without artificial encapsulated shrimp larvae feed.
Dead larvae and uneaten food were siphoned out from the tanks daily. Larval numbers are estimated and monitored daily through volumetric using 5 mL pipette from the rearing tanks. Water quality parameters such as temperature salinity, pH and dissolved oxygen were recorded. The water culture media temperature was maintained at 30˚C using 3 phase thermostat water heater.
The larvae rearing trial will end when all the crab larvae within the culture rearing tank metamorphosed to 1st day juvenile crabs (C1). Larvae were fed once daily in the morning at about 10.00 hr throughout the study trials. Moderate aeration was provided in the larval rearing tanks. The summary of the feeding regimes experimental design are as in Table 1.
2.2. Data Collection
The main data collected during the study trial were survival rate of larvae stage till the 1st day juvenile crab. Any 1st day juvenile crab (C1) present was removed from the larvae rearing tanks at the time of the daily count. Trials were terminated when all the larvae had meta-

Table 1. Summary of the different diets throughout the study periods for P. pelagicus larvae.
morphosed to crabs or died. The survival rates to C1 were expressed as percent of the initial number of larvae at zoea 1 stage stocked into the larvae rearing tank.
2.3. Data Analysis
Data are presented as mean ± SD. Statistical significance of differences among treatments for survival rate from zoea 1 (Z1) to 1st day juvenile crab (C1) was determined using Analysis of Variance. The Analysis of Variance of data was used to test for; 1) larval rearing treated with frozen Nannochloropsis oculata (batches no. 1) and without frozen Nannochloropsis oculata (batches no. 2), 2) larval rearing treated with Spirulina (batches no. 3) and without Spirulina (batches no. 4) and 3) larval rearing treated with shrimp larvae feed (batches no. 5) and without shrimp larvae feed (batches no. 6). A Fixed-Ratio Hypothesis of Chi-Square Test was used to test for; 1) the number of larvae rearing batches reached megalopa stage within 12 days as compared to other days of 11 days, 13 days and 14 days and, 2) the number of larvae rearing batches reached 1st day crab stage within 17 days as compared to other days.
3. RESULTS
3.1. Treatment with and without Instant Frozen Nannochloropsis oculata
The results of this trial revealed that a combination diet of instant frozen Nannochloropsis oculata, Artemia nauplii and rotifer gives a better survival rate till 1st day juvenile crab of 1.49% for one of the three replicates (larvae rearing batch No. 1a). However the other two replicate were still producing poor results with survival rate till 1st day juvenile crab was 0.11% and 0.01% for larvae rearing batch No. 1b and 1c respectively (Table 2).
By the end of zoea 3 of the 9th day, survival rate was

Table 2. Survival rate and moult time of larvae stage till the 1st day juvenile crab of P. pelagicus larvae fed with combination of instant frozen Nannochloropsis oculata, rotifer and Artemia nauplii for larvae rearing batch No. 1 (1a, 1b and 1c) and without instant frozen Nannochloropsis oculata but fed only with rotifer and Artemia nauplii larvae rearing batch No. 2 (2a, 2b and 2c).
reduced further to less than 50%. The same very low survival rate till 1st day juvenile crab was recorded for the control treatment as compare to previous experiment which was also fed with the combination of Artemia nauplii and rotifer. The survival rate recorded was 0.002%, 0.001% and 0.004% for larvae rearing batch No. 2a, 2b and 2c respectively (Table 2). Analysis of variance also shows that there was no significant difference in the survival rate of zoea 1 to 1st day juvenile crab when testing the mean survival rate effect of the larvae feed with instant frozen Nannochloropsis oculata and without instant frozen Nannochloropsis oculata. In all the larvae rearing experiments (larvae rearing batch No. 1a, 1b, 1c, 2a, 2b and 2c), the study shows that the zoea reached the megalopa stage in 12 days and reached the C1 in 17 days. In this experiment, the results also show that the moulting was synchronous within all treatment and control batches.
3.2. Treatment with and without Encapsulated Spirulina
The results of this trial also produced similar pattern as the previous treatment with and without instant frozen Nannochloropsis oculata with a bit lower survival rate. The survival rate till 1st day juvenile crab feed using combination of encapsulated Spirulina, Artemia nauplii and rotifer is 0.29%, 0.01% and 0.31% for larvae rearing batch No. 3a, 3b and 3c (Table 3). By the end of zoea 3 of the 9th day, survival rate was reduced further to less than 10% (Table 3). The low survival rate till 1st day juvenile crab was still recorded for the control treatment as compare to two previous experiment trials (larvae rearing batch No. 2a, 2b, 2c) which was also feed with the combination of Artemia nauplii and rotifer. The survival rate recorded was 0.01%, 0.003% and 0.01% for larvae rearing batch No. 4a, 4b and 4c respectively (Table 3). Analysis also shows that there was no significant difference in the survival rate of zoea 1 to 1st day juvenile crab when testing the mean survival rate effect of the larvae fed with encapsulated Spirulina and without encapsulated Spirulina. The results from the experiments treated with and without encapsulated Spirulina shows that the zoea reached the megalopa stage within 13 to 14 days which is 1 to 2 days longer as compared to previous

Table 3. Survival rate and moult time of larvae stage till the 1st day juvenile crab of P. pelagicus larvae fed with combination of encapsulated Spirulina, rotifer and Artemia nauplii for larvae rearing batch No. 3 (3a, 3b and 3c) and without encapsulated Spirulina but fed only with rotifer and Artemia nauplii for larvae rearing batch No. 4 (4a, 4b and 4c).
trials treated with and without instant frozen Nannochloropsis oculata. An experiment treated with encapsulated Spirulina recorded only 16 days for the zoea to reach the C1, one day earlier as compared to previous trials treated with and without instant frozen Nannochloropsis oculata. The results also show that the moulting was not synchronized within all treatment and control batches.
3.3. Treatment with and without Artificial Encapsulated Shrimp Larvae Feed
The results of this trial also have similar pattern as the previous two treatments with and without instant frozen Nannochloropsis oculata and encapsulated Spirulina. The survival rate till 1st day juvenile crab fed with combination of artificial encapsulated shrimp larvae feed, Artemia nauplii and rotifer were 0.02%, 0.45% and 0.1% for larvae rearing batch No. 5a, 5b and 5c (Table 4). By the end of zoea 1 of the 5th day, survival rate was reduced further to less than 50% for larvae rearing batch No. 5a and 5b (Table 4). However, it took 10 days within zoea 3 stage before survival rate to less than 50% for larvae rearing batch No. 5c (Table 4). The same very low survival rate till 1st day juvenile crab was still recorded for the control treatment as compare to three previous experiment trials (larvae rearing batch No. 2a, 2b, 2c, 4a, 4b and 4c) which was also feed with the combination of Artemia nauplii and rotifer. The survival rate recorded was 0.01%, 0.001% and 0.001% for larvae rearing batch No. 6a, 6b and 6c respectively (Table 4). Analysis of variance also shows that there was no significant difference in the survival rate of zoea 1 to 1st day juvenile crab when testing the mean survival rate effect of the larvae feed with artificial encapsulated shrimp larvae feed and without artificial encapsulated shrimp larvae feed. The results from the experiments treated with and without artificial encapsulated shrimp larvae feed shows that the zoea 1 took 5 days before zoea 2 stages were reached, which 1 to 2 days longer as compared to all the previous experiment trials earlier. The results also shows that zoea 1 reached the megalopa stage within 14 days which is 1 day longer as compared to previous trials treated with and without instant frozen Nannochloropsis oculata. One of experiment treated with artificial encapsulated shrimp
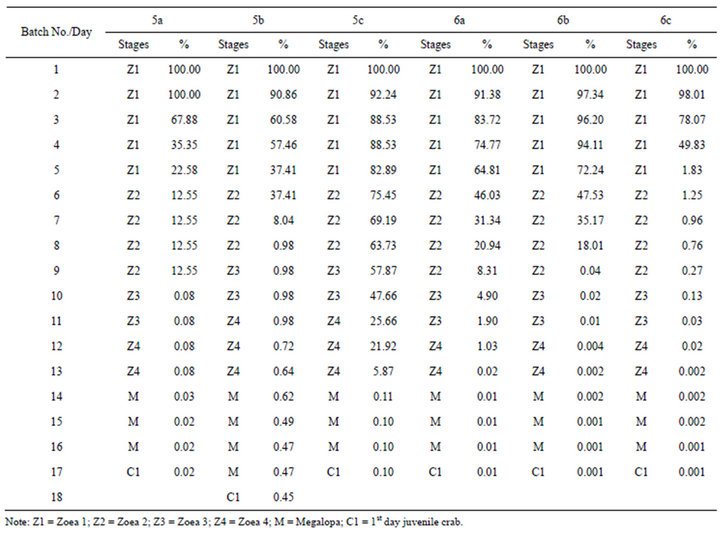
Table 4. Survival rate and moult time of larvae stage till the 1st day juvenile crab of P. pelagicus larvae fed with combination of artificial encapsulated shrimp larvae feed, rotifer and Artemia nauplii for larvae rearing batch No. 5 (5a, 5b and 5c) and without artificial encapsulated shrimp larvae feed but fed only with rotifer and Artemia nauplii for larvae rearing batch No. 6 (6a, 6b and 6c).
larvae feed (larvae rearing batch No. 5b) recorded 18 days for the zoea to reach the C1, 1 to 2 days longer as compared to all the previous trials earlier (Table 4).
3.4. Growth of Crab Larvae
The results of the present study show that the zoea reached the megalopa stage between 11 - 14 days; most zoea reached the megalopa stage at day 12. However for the zoea to reached the C1 stages was between 16 - 18 days; most zoea reached the C1 stage at day 17 (Table 5). The above results are based on the mean daily water parameter reading through the study trial at 28.77˚C for temperature, 31.91 ppt for salinity, 8.66 for pH and 7.67 mg/l for dissolved oxygen.
4. DISCUSSIONS
The results of the present study concluded that a combination diet of rotifer and Artemia nauplii is ideal for rearing of P. pelagicus with rotifer is recommended for use at early zoea stage (zoea 1 to zoea 2) and Artemia

Table 5. Number of days for P. pelagicus crab larvae reached megalopa and C1 stages from 18 experiment trials of 6 larvae rearing batches with 3 replicates each of the feeding regimes study trial.
nauplii is recommended for use at late zoea stage (zoea 3 to zoea 4) till the C1 stage.
Larvae rearing of P. pelagicus were also carried out to find out if adding Nannochloropsis and Spirulina on the culture water would improve survival of the crab larvae from zoea 1 stage up to C1, using rotifer and Artemia as the main food items (larvae rearing batch no. 1, 7 and 9). It have been reported that phytoplankton added to the culture water seemed to have a “beneficial” effect in larval fish cultures in terms of survival by releasing oxygen into and removing certain metabolites like ammonia, from the culture medium [16]. It was even suggested that phytoplankton also releases antibiotic substance into the culture medium [16]. The results of the present study also shows that there are no significant different in larval survival till C1, in treatments with and without diatom (larvae rearing batch no. 1 and 2), with and without instant frozen Nannochloropsis oculata (larvae rearing batch no. 7 and 8), and with and without encapsulated Spirulina (larvae rearing batch no. 9 and 10). The results of the present study concluded that the adding of phytoplankton (i.e. diatom, Nannochloropsis and Spirulina) to larvae rearing system where rotifer and Artemia nauplii is main food items did not produced high survival rate as compared to larvae rearing fed on rotifer and Artemia nauplii alone.
Other than phytoplankton, artificial encapsulated shrimp larvae feed were also added on the culture water to improve the survival of the crab larvae from zoea 1 stage up to C1, using rotifer and Artemia nauplii as the main food items (larvae rearing batch no. 11). Artificial diet could serve as enrichment for rotifer and Artemia nauplii, which in turn are taken in by the crab larvae. The supplementation of artificial diets could improve the growth and survival of crab larvae and reduce the requirement for natural food. However the results of the present study also concluded that the adding of artificial diet to larvae rearing system where rotifer and Artemia nauplii is main food items (larvae rearing batch no. 11) did not produced high survival rate as compared to larvae rearing fed on rotifer and Artemia nauplii alone (larvae rearing batch no. 12). The similar low survival for both treated with and without artificial diet may deal to increased particle sedimentation leading to water deterioration and increased in bacterial load. This was also been proved by Quinitio et al. [17] where high concentrations of luminous Vibrio were detected in rearing water for replicates treated with artificial diet (3.5 × 102 to 2.5 × 103/cfu/ml) and counts were less than 1 × 102/cfu/ml or sometimes not detectable in the larvae rearing without using artificial diet.
The results of the present study demonstrated that the food types not only affect survival rate but also the growth of crab larvae (i.e. moult times). These are also supported by other studies on mangrove crab [16-18]. Study by Nguyen and Truong [18] shows that the food types affect both the survival rate and growth of mangrove crab larvae (S. paramamosain). Study by Quinitio et al. [17] shows that the supplementation of artificial diets not only could improve the survival but also the growth of the mangrove crab larvae (S. serrata). Studies by Jones et al. [19] and Kanazawa et al. [20] reported that survival and growth of Penaeid shrimp larvae were greatly improved by enriching rotifers and Artemia nauplii with essential HUFA.
5. CONCLUSION
Food type influenced survival, development, and metamorphosis to megalopa and 1st day juvenile crab of P. pelagicus zoea larvae. The best survival, the most rapid development and the highest number of 1st day juvenile crabs produced were obtained from larvae fed with a combination diet of frozen N. oculata, Artemia nauplii and rotifer from hatching till the 1st day juvenile crab. The present study generally concluded that the combination diet of N. oculata, rotifer, Artemia nauplii alone is enough to produce 1st day juvenile crabs as compared with adding any artificial diet to the culture rearing system.
6. ACKNOWLEDGEMENTS
This work was supported by IRPA R&D grant from Ministry of Science, Technology and Innovation, Malaysia. We would like also to thanks the Inland Fisheries, Agriculture Department, Sarawak, Malaysia for assisting in the laboratory and field works and, the Faculty of Science and Resource Technology, Universiti Malaysia Sarawak, Malaysia for their technical support.