Combination of Diclofenac Potassium and Propolis in the Therapy of Oral Aphthosis: A Randomized, Clinical, Double-Blind, Placebo-Controlled Study ()
1. Introduction
The oral mucous tissue disease known as an aphtha is characterized by the presence of an excruciatingly painful ulcer. In various locations across the oral cavity, they can be found alone or in groups. It initially causes itching or burning in the mouth, where a lesion will develop in a day or two [1] . A differential diagnosis must be made to rule out aphthous-like lesions such as herpes, autoimmune diseases, and pediatric infections [2] .
Oral aphthous has no confirmed causes, even though it appears likely to be a disorder of the oral cavity’s bacterial flora linked to immune system issues [3] [4] [5] . Specific antigens in the major histocompatibility complex make some people more genetically susceptible to aphthae [6] [7] . Other pathogenesis-related mechanisms and factors for aphthous ulcers include intercellular edema and degenerative changes, intra-nuclear inclusion bodies, IgG and IgM, an increase in mast cells [8] [9] [10] . Aphthous ulcers are difficult to cure and control [11] , Different therapy options included pharmacological and non-pharmacological approaches [12] . Diclofenac potassium was anticipated to have high anti-inflammatory effects when given topically due to its good penetration capabilities and strong suppression of PGE2 production [13] [14] .
Greek and Roman physicians Aristoteles, Dioscorides, Pliny, and Galen recognized the extraordinary safety profile of propolis and its use by the Egyptians knew to embalm bodies, as well as its anti-inflammatory and immunoregulatory qualities as prospective treatments for SARS-CoV-2 infection and COVID-19 [15] [16] [17] . Aphthous stomatitis and mouth ulcers may be managed with the help of propolis [18] .
2. Patients and Methods
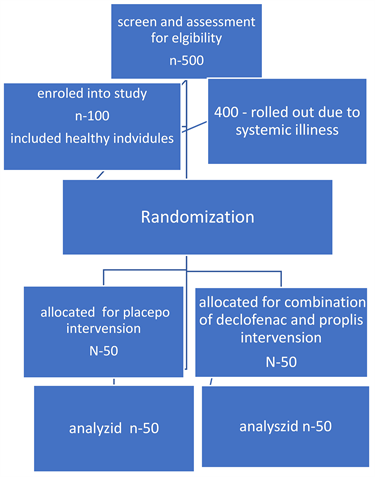
The investigation was planned as a clinical, double-blind, placebo-controlled randomized trial. After screening 500 subjects for eligibility, 400 were disqualified due to systemic illnesses, and 100 healthy volunteers with aphthous ulcers qualified for the study’s specific conditions. They were selected from an outpatient clinic at the Department of Oral Medicine, Periodontology, Diagnosis, and Oral Radiology, Faculty of Dental Medicine (Boys-Cairo), Al-Azhar University.
Eligibility criteria of the population: Patients with systemic disease were excluded, in addition to those who were allergic to any ingredient in a formulation containing diclofenac potassium, as well as those who were using other NSAIDs.
Interventions: According to a computer-generated list of random numbers, an independent clinician randomly distributed either active or placebo tubes. (Group 1): fifty individuals received a placebo. And the other group received treatment with a combination of diclofenac potassium 3% (10 mg/g, 60 g) and propolis 5% gel prepared by the condensation method at the Therapeutic Chemistry Department, National Research Centre, Giza, Egypt. (Group II). fifty individuals, as in the following flow chart. Over 10 days, the patients were instructed to apply the gel twice daily. After applying the gel, the patients were advised to wait one hour before eating or drinking.
Flow chart
Observations: Participants were evaluated for symptomatic reduction in pain, size, healing length, frequency of recurrence, and number of ulcers on day 1, day 3, day 5, day 7, and after healing. For follow-up, a digital photo was recorded every five days using the same camera, the same lighting, and the same distance. A score was assigned to the photos based on how the ulcer appeared clinically after treatment: 1-complete improvement, 2-partial improvement, 3-no change, and 4-worsening of clinical presentation. For the evaluation of the tolerability of the drug and patient satisfaction, a questionnaire (the Chronic Oral Mucosal Diseases Questionnaire) adapted from the study of Nelson and Spencer was applied at the end of treatment using a 5-point scale of responses, where 0 = I neither agree nor disagree; 1 = I slightly agree; 2 = I completely agree; 3 = I slightly disagree; and 4 = I completely disagree, to the following statements: 1) My ulcer lesions have improved with this product; 2) This product does not cause irritation to the mouth; 3) I am satisfied with this product.
Numerical data were explored for normality by checking the distribution of the data and using tests of normality (the Kolmogorov-Smirnov and Shapiro-Wilk tests). The distribution of age data was normal (parametric), whereas the distribution of quality-of-life scores was non-parametric. The data were presented as median, range, mean, and standard deviation (SD) values. For parametric data, a student’s t-test was used to compare the mean age values in the two groups. For non-parametric data, the Mann-Whitney U test was used to compare the two groups. Friedman’s test was used to study the changes within each group. Dunn’s test was used for pair-wise comparisons when Friedman’s test was significant. Qualitative data (gender) was presented as frequencies and percentages. The Chi-square test was used to compare the two groups. The significance level was set at P ≤ 0.05. Statistical analysis was performed with IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.
Ethical Approval:
The nature of the study has been explained to patients. Upon their agreement, they signed a written consent form. The uses of human data were done in conformity with the Helsinki Declaration. The faculty ethical committee signed the study acceptance with the code 650/3607.
3. Results
The comprehensive quality-of-life survey for chronic oral mucosal illnesses showed that Group I received a statistically significantly lower median total score than Group II before treatment. In comparison to Group II, Group I had a statistically significantly higher median total score at one, three, five, seven, and after healing. There was a statistically significant change over time in Group I with reference to the changes over time. The median total score showed a statistically significant decline after one day, from one to three and from three to five days, followed by non-statistically significant results for the remaining follow-up periods, according to pair-wise comparisons between time periods. Regarding the changes over time within Group II, a statistically significant change over time was observed. The median total score had a statistically significant decline after one day and from one to three days, followed by non-statistically significant change for the remainder of the follow-up periods, according to pair-wise comparisons between time periods (Table 1).
![]()
Table 1. Descriptive statistics, results of Mann-Whitney U test for comparison between total scores of chronic Oral Mucosal Diseases Questionnaire in the two groups and Friedman’s test for the changes within each group.
*: Significant at P ≤ 0.05, Different superscripts in the same column indicate statistically significant changes within group.
There was a statistically significant difference between the two groups in terms of ulcer improvement with treatments, according to patient satisfaction questionnaire results and drug tolerance. The percentage of complete or slight agreement was higher in Group II than in Group I. There was a statistically significant difference between the two groups in mouth irritation. More people in Group II than in Group I agreed completely or somewhat that the medicine doesn’t cause irritation. As regards satisfaction with the product, there was a statistically significant difference between the two groups. Group II showed a higher percentage of complete or slight satisfaction than Group I (Table 2).
According to the clinical photo score, Group II demonstrated 48% complete healing and 52% partial healing after one day, 88% complete healing after three days, 96% complete healing after five days, and 100% complete recovery at seven days. While group I demonstrated a 4% partial healing and 96% no change after one day, 90% no change after three days, 2% complete healing after five days, and 2% clinical appearance worsening after seven days (Figures 1-7).
![]()
Figure 1. A bar graph representing the percentages and results of the Chi-square test for comparison of drug tolerability and patient satisfaction about the medication in the two groups.
![]()
Figure 2. Clinical photos represent palatal ulcer treated with the combination of diclofenac potassium and propolis. A: Before gel application; B: One day after application; C: 3-day after application.
![]()
Table 2. Frequencies (n), percentages and results of Chi-square test for comparison between answers to the questionnaire about evaluation of tolerability of the drug and patient satisfaction in the two groups.
*: Significant at P ≤ 0.0.
![]()
Figure 3. Bar graph showing the score of clinical photos in the two groups at one day after gel application (Group I: The placebo group; Group II: The combination group).
![]()
Figure 4. Bar graph showing the score of clinical photos in the two groups at 7 days after gel application.
![]()
Figure 5. Clinical photos represent Aphthous ulcer at the lower lip showing rapid healing after one day combination gel had been applied. A: Before gel application; B: One day after gel application.
![]()
Figure 6. Clinical photos show after one day, aphthous ulcers at the buccal mucosa shrank in size. A: A day before applying the gel; B: A day after applying the gel.
4. Discussions
The acute discomfort imposed by aphthous ulcers has been treated using a variety of techniques and preparation forms for RAS treatment that contain both active ingredients and excipients [19] . Patients have not yet received a therapy
![]()
Figure 7. Clinical photos show the size and intensity of aphthous ulcer within the labial mucobuccal fold have decreased. A: Before applying gel; B: One day after applying gel.
regimen that is immediate, efficient, has few adverse effects, and is affordable. It is difficult to treat these lesions clinically. Pain is the most credible factor impacting patients with oral ulcers. In contrast to previous studies that showed pain relief taking a long time, this study showed immediate pain relief. According to several studies, low-level laser therapy is an effective alternative or supplemental RAS treatment. Considering that the exact cause of RAS is unknown [20] .
According to this study, ulcer healing rates have changed by 100%, going from 54% full healing to 46% partial healing, which has resulted in the shortest healing times, most discomfort, and largest ulcer sizes ever recorded. This was preferable to using steroid pills, which are one of the most widely used therapies in specialized clinics [21] [22] . In terms of speeding up the healing process [23] , Amlexanox oral adhesive tablets were successful at promoting healing and minimizing discomfort when applied four times daily for five days in two respectably sized double-blind trials (100 - 200 RAS patients) [24] . Topical tetracycline or minocycline mouthwashes as a local antibacterial treatment for major and minor RAS are expected to lessen the severity of the ulcerations and pain but not to stop recurrences [10] . Comparable effectiveness in promoting mending and reducing pain has been reported with penicillin-G mouthwashes, Aloe vera gel is one of the topical herbal remedies that has demonstrated effectiveness as an alternative therapy [25] . Systemic prednisolone, dapsone, levamisole, azathioprine, pentoxifylline, colchicine, and thalidomide have been studied for the management of severe and recurrent RAS. They all have side effects and take a while to reduce pain, in contrast to the findings of this study, which showed that there are no side effects, and that pain relief occurs instantly. As a result, the severity of the RAS and any possible drug side effects should be taken into consideration while choosing a course of treatment [26] .
Levamisole alone is an effective treatment, reducing ulcers by up to 66% [27] , Infliximab, adalimumab, and golimumab Approximately 89% of patients have made a full recovery [28] [29] . In this trial, the active sample performed much better. This could be due to the unique analgesic and potent anti-inflammatory benefits of the combination gel, which have been seen throughout this brief follow-up period without causing any negative effects.
5. Conclusion
The combination of diclofenac and propolis may offer a different approach to treating recurring aphthous ulcers, as well as instant symptom alleviation, improved quality of life, and a safe and innovative, cost-effective regimen for treating disorders involving oral ulcers.
6. Limitations
There are certain limitations that could be addressed. The study focused on subjective photo evaluation. In future it could be scored using automatic methods.