The Use of Intramuscular Praziquantel in South-African Ruminants against Cestode Parasites. A First Pilot Study ()
1. Introduction
Attempting to increase the animal productivity and improving quality of life in the animal herd and efforts to farm more economically in South Africa, are all reasons to explore more superior methods of managing the health of production herds. Internal parasites pose a threat to these objectives of the modern farmer and this paper aims at exploring the potential use of injectable Praziquantel on small ruminants. If proven successful, further trials and research on this application in the wildlife industry may revolutionize Cestode treatment and management in wildlife populations in Sub-Saharan Africa.
Apart from tapeworms depriving the host animal from nutrients, Cestodes also present a threat to human health, as people may inadvertently become unwanted unintended intermediate hosts or final hosts to these parasites, each situation presenting its own clinical medical risks. There has also been a suggestion that enterotoxemia (overeating disease) caused by Clostridium bacteria may occur more often in animals with heavy tapeworm infection. There have been no experimental studies to support this hypothesis, but researchers find that a common rat tapeworm reduced the muscular activity of the small intestine of infected rats and this change was associated with a change in the species of bacteria in the intestine. Possibly Moniezia could have a similar effect in the small ruminant intestine and lead to increased rates of bacterial disease in the presence of other specific nutritional or environmental conditions that haven’t been identified. It is the aim of this trial study to determine if the use of injectable Praziquantel is effective against tapeworm infestations in goats.
2. The Research Objective
The following objectives are set for the project:
1) Determine the intestinal parasite load of Nematode and Cestode helminths in the test herds and control herds of ruminants.
2) Divide each herd into two sub herds to enable product and dose comparison with a control herd for each.
3) Treat each herd and sub herd according to the prescribed and established dose per remedy group.
4) Evaluate the efficacy of each herd treatment of the Praziquantel group to that of the associated control herd.
5) Provide recommendations in terms of the use of Praziquantel and associated optimum doses.
3. The Study Area
The first study area is the farm Lekkersmaak, located 30 Km west from the town of Phalaborwa, Limpopo province, South Africa. The GPS coordinates are 1,234,567,890S, 1,234,567,890E. The farm utilizes an open grazing system on natural pasture comprising 400 Ha of mixed Mopaniveld. The goats return to the kraal at the homestead every evening.
The second study area is the farm Selwane, located 35 Km North-west from the town of Phalaborwa, Limpopo province, South Africa. The GPS coordinates are 23,456,789S, 23,456,789E. The farm also utilizes an open grazing system on natural pasture comprising 800 Ha of mixed Mopaniveld. The goats also return to the kraal at the homestead every evening.
4. Review of Related Literature
This chapter contains information on Praziquantel Injection for veterinary use.
The information provided typically includes the following: (Obtained from Kyron Prescriptions)
· Praziquantel Injection Indications
· Warnings and cautions for Praziquantel Injection
· Direction and dosage information for Praziquantel Injection
INJECTABLE CESTOCIDE FOR DOGS AND CATS 56.8 mg/mL Solution
NADA 111-607, Approved by FDA CONTAINS PER ML: Praziquantel 56.8 mg, benzyl alcohol 75 mg, chlorobutanol hydrous 5 mg in propylene glycol q.s.
Description
Praziquantel Injection Cestodicide is a clear solution containing 56.8 milligrams of Praziquantel per mL which has been formulated for subcutaneous or intramuscular use in dogs and cats for removal of Cestodes (tapeworms).
Praziquantel Injection Indications
Praziquantel Injection Cestodicide is indicated for the removal of the following canine and/or feline Cestodes [1].
Dogs: Dipylidium caninum, Taenia pisiformis, Echinococcus granulosus and for the removal and control of Echinococcus multilocularis.
Cats: Taenia taeniaeformis and Dipylidium caninum.
Action:
Praziquantel Injection is absorbed, metabolized in the liver and excreted via the bile into the digestive tract where its cestocidal activity is exerted. Following exposure to Praziquantel, the tapeworm loses its ability to resist digestion by the mammalian host. Because of this, whole tapeworms, including the scolex, are very rarely passed after administration of Praziquantel. It is common to see only disintegrated and partially digested pieces of tapeworms in the stool. The majority of tapeworms killed is digested and are not found in the feces.
Use directions:
Praziquantel Injection Cestodicide may be administered by either the subcutaneous or intramuscular route to dogs and cats. The recommended dosage of Praziquantel varies according to body weight. Smaller animals require a relatively larger dosage. The optimum dosage for each individual animal will be achieved by utilizing the following dosage schedule. The recommended dosage of Praziquantel is not affected by the presence or absence of food in the gastrointestinal tract.
Administration:
Praziquantel Injection Cestodicide may be administered by either the subcutaneous or intramuscular route to dogs and cats. The intramuscular route may be preferred in dogs due to a brief period of pain that occasionally follows subcutaneous administration. Anaphylactoid reactions were not observed in clinical trials. However, as with any drug an anaphylactoid reaction can occur with this product and should be treated symptomatically if it occurs.
Re-treatment:
For those animals living where reinfections are likely to occur, clients should be instructed in the steps to optimize prevention, otherwise, Re-treatment may be necessary. This is true in cases of Dipylidium caninum where reinfection is almost certain to occur if fleas are not removed from the animal and its environment. In addition, for control of Echinococcus multilocularis, a program of regular treatment every 21 to 26 days may be indicated (see E. multilocularis section below).
Diagnosis:
Diagnosis of E. multilocularis in canids is difficult. The adult tapeworm produces no clinical signs of infection. Tapeworm segments (proglottids) are usually not observed in the feces. E. multilocularis eggs, observed using microscopic fecal examination procedures, are similar in appearance to the common taeniid species of canids such as Taenia pisiformis. Assistance in the diagnosis of E. multilocularis may be available from a diagnostic laboratory.
Treatment:
Dogs infected with E. multilocularis should be treated to prevent exposure of humans to infective eggs and to reduce perpetuation of the parasite’s life cycle [2].
The dosage of Praziquantel Injection solution for removal of E. multilocularis is the same as that indicated for the removal of the other tapeworm species listed on the label [3]. Laboratory efficacy studies have demonstrated the recommended dosage is 100% efficacious for removal of this tapeworm. Under condition of continual exposure to wild rodents, Re-treatment of the dog at 21 - 26 day intervals is recommended to prevent the shedding of infectious eggs.
Precautions:
Strict hygienic precautions should be taken when handling dogs or feces suspected of harboring E. multilocularis. Infected dogs treated for the first time with Praziquantel Injection solution and dogs treated at intervals greater than 28 days may shed eggs in the feces after treatment. The animal should be held in the clinic during this interval and all feces should be incinerated or autoclaved. If these procedures are not possible, the eggs can be destroyed by soaking the feces in a sodium hypochlorite (bleach) solution of 3.75% or greater. All areas where the animal was maintained or in contact with, should be thoroughly cleaned with sodium hypochlorite and allowed to dry completely before reuse.
Overdosage:
The safety index has been derived from controlled safety evaluations, clinical trials and prior approved use in foreign countries. Dosages of 5 times the labeled rate at 14 day intervals to dogs as young as 4 weeks did not produce signs of clinical toxicity following either intramuscular or subcutaneous injections. No significant clinical chemistry, hematological, cholinesterase or histopathological changes occurred. Dosages of 5 times the labeled rate at 14 day intervals to kittens as young as 5 1/2 weeks did not produce signs of clinical toxicity following either intramuscular or subcutaneous injections. Symptoms of overdosage (33.8 to 40 times the labeled dosage rate) in adult dogs included vomition, excessive salivation and depression, but no deaths. Symptoms of overdosage (10 to 20 times the labeled dosage rate) in adult cats included vomition, depression, muscle tremors and incoordination. Deaths occurred in 5 of 8 cats treated subcutaneously and in all 8 injected intramuscularly at doses greater than 20 times the label rate.
Contraindications:
There are no known contraindications to the use of Praziquantel.
Pregnancy:
Praziquantel Injection has been tested in breeding and pregnant dogs and cats. No adverse effects were noted.
Adverse Reactions:
Mild side effects were observed in 18 of 189 dogs (9.5%) and 8 of 85 cats (9.4%) administered Praziquantel Injection in field trials. For dogs the majority of these were described as brief pain responses following injections to larger dogs (weighing over 50 lbs.). Two dogs exhibited a brief period of mild vomiting and/or drowsy or staggering gait. The eight cats exhibited either diarrhea, weakness, vomition, salivation, sleepiness, burning on injection and/or a temporary lack of appetite. Local irritation or swelling at the site of subcutaneous injections have been reported for cats.
Summary of product:
Praziquantel is an excellent taenicide belonging to the chemical class of the isoquinolines. It is very much used in dogs and cats in various delivery forms (e.g. suspensions, injectables, tablets, pills, etc.). It is very much less used in livestock (a few products for sheep) or birds. Due to its narrow spectrum of activity it is often used on pets in combination with a broad spectrum nematicide (e.g. macrocyclic lactones, benzimidazoles, tetrahydropyrimidines, etc.)
Efficacy of Praziquantel
Praziquantel is highly effective against adults of many parasitic tapeworms (i.e. Cestodes, e.g. of the genus Dipylidium, Taenia, Echinococcus, Mesocestoides, Moniezia, Avitellina, Stilesia, etc.) as well as against a few flukes (i.e. trematodes, e.g. of the genus Eurytrema and Schistosoma). It is also effective against larvae and/or eggs of certain species [4].
The delivery form can influence the efficacy against certain parasites: e.g. efficacy against Echinococcus is usually lower after subcutaneous injection than after oral administration. It has no efficacy whatsoever against roundworms (nematodes) and other important parasitic flukes such as liver flukes (Fasciola spp) or stomach flukes (Paramphistomum spp), nor against external parasites [5]. Praziquantel has no residual effect. This means that a single administration will kill the parasites present in the host at the time of treatment, but will not protect the host against re-infestations.
Pharmacokinetics of Praziquantel
After oral or parenteral (injection) administration Praziquantel is quickly and almost completely absorbed into the bloodstream in all species. Maximum plasma levels are reached 30 to 120 minutes after administration in dogs, 2 hours after administration in sheep. It is also quickly distributed throughout the whole body: highest concentrations are found in the liver and the kidneys. It is partly released back to the gut lumen, which makes it effective against parasitic stages in the intestinal wall. Praziquantel is quickly metabolized to ineffective metabolites. Half-life in plasma is about 30 minutes, i.e., the anthelmintic effect is rather brief and hence it's lack of residual effect. Excretion is accomplished 40% to 70% through urine, the rest through bile and feces, almost completely in the form of its metabolites. Less than 1% is excreted unchanged. Excretion half-life in dogs and sheep is 2 to 3 hours. Twenty-four hours after treatment 80% of the administered dose has been excreted, 48 hours after treatment excretion is complete.
Mechanism of action of Praziquantel:
The molecular mode of action of Praziquantel is not precisely known at present. In Schistosoma worms it seems to affect the permeability of the calcium ions in the muscular membrane. As consequence the worms are paralyzed and die. In tapeworms it seems to affect carbohydrate metabolism and to damage the worm's tegument structures. As a consequence the parasites cannot avoid being digested by the gastric fluids of the host.
5. The Methods and Materials
The herds from the two study areas were divided as follows:
21 to 25 Goats per farm were randomly selected for the trial, and two camps were erected on each farm for the experiment, named respectively:
Lekkersmaak farm:
Praziquantel camp: 11 goats
Control camp: 10 goats
Selwane farm:
Praziquantel camp: 15 goats
Control camp: 10 goats
In total, 46 goats were selected for this trial between the two study areas.
The following metodology was adopted starting in sequence on day 0 (zero):
· Each goat was fitted with an ear tag and unique identity number. Praziquantel goats were fitted with a red ear tag and control goats were fitted with a white ear tag.
· Identity numbers were allocated according to location, camp and goat number. Lekkersmaak farm goats were allocated a prefix of the letter “L”, followed by the letter representing the camp (P = Praziquantel or C = Control) and finally followed by the number of goat in that camp, for example 1. Final typical ear tag ID would look typically as follows: LP3, which would indicate a goat from Lekkersmaak, representing the Praziquantel camp and it is goat number 3 of that camp. A similar system is to be used on Selwane farm, with the S indicating the different farm.
· A fecal sample was collected from the rectum of each animal. The samples were bagged, sealed, labeled, cooled and taken to the laboratory for coprological examination for Nematodes and Cestodes.
· Each animal was divided into weight/size classes, namely large adult (±180 pd), medium sub-adult or ewe (100 pd) and small yearling (±60 pd).
· Following, each animal received the prescribed treatment dictated by the methodology and dose recommendations from the manufacturer, per weight class.
Praziquantel camps:
The Praziquantel was injected intramuscular at an average of 3 mL per goat, working on an average of 30 Kg weight per goat. This translates to 1 mL/10 Kg. The Praziquantel received for this trial had a concentration of 5.68% (56.8 mg/mL). This implies that the 100 mL bottle received, contained 5680 mg of Praziquantel.
At 56.8 mg/mL, and with an average weight of 30 kg, 3 mL of Praziquantel translates into a final proposed trial dose of 5.68 mg/Kg. This corresponds with the prescribed SC administration of Praziquantel in cats and dogs [6].
Control camps:
· The goats were kept on kraal for 3 days after treatment, as the Praziquantel is completely metabolized within that time. The animals were fed during those 3 days in order to prevent immediate reinfection when grazing in the open pasture of the farm and the herds mixing again.
· On day 3, fecal samples were collected once more and sent to the laboratory for coprological examination to determine the post treatment egg count of parasites.
6. Results and Discussion
Below are the results from the pre- and post treatment of the goats using Praziquantel and with a control of no treatment at all. As the sample populations were relatively small, it was decided to keep statistical analysis basic in order to prevent distortion of the data due to possible statistical type one errors, associated with under-sampling.
Data is provided per farm indicating EPG results (Eggs per gram of feces) for both pre-treatment as well as post treatment as shown by Tables 1-4. These results are summarized in Chart 1.
Interpretation:
Moniezia spp egg count decreased significantly after treatment. It is acknowledged that some of the test goats had escaped and could not be included again after capture, as re-infecion could not be ruled out. This could also have influenced the observed decrease in EPG in the test herd. This having been said, it is clear that numbers of Coccidia and Haemonchus increased sharply even post treatment. This supports the fact that Praziquantel is not effective against Nematodes.
Interpretation:
When only considering the Moniezia spp eggs to calculate EPG, it also demonstrates a high level of efficacy of the Praziquantel against these helminths. Not without sample fault as mentioned before, Praziquantel still produced 100% reduction in eggs, while Moniezia egg counts stayed virtually unchanged on herd level in the control group, but significant increases in egg numbers did occur at individual level.
![]()
Table 1. Selwane farm results: Pre- and post treatment with praziquantel, including Cestode, Nematode and Coccidia.
Interpretation:
Lekkersmaak farm data followed the same pattern as that of Selwane farm herd.
Interpretation:
Praziquantel treatment resulted in a reduction in Moniezia eggs observed, by up to 84.6% after treatment, while an increase of 8.6% eggs produced within 3 days was observed in he control herd where no treatment was given.
![]()
Table 2. Selwane farm results: Pre- and post treatment with praziquantel for Cestode only.
![]()
Table 3. Lekkersmaak farm results: Pre- and post treatment with praziquantel, including Cestode, Nematode and Coccidia.
![]()
Table 4. Lekkersmaak farm results: Pre- and post treatment with praziquantel for Cestode only.
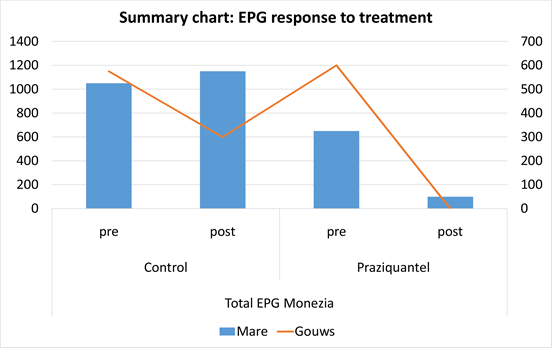
Chart 1. Response to treatment with Praziquantel based on egg counts before and after treatment, compare to the control egg counts where no treatment was applied.
7. Conclusion
On averages, Lekkersmaak farm experienced a Moniezia reduction of 69% within 3 days after treatment with injectable Praziquantel. Farm Selwane experienced an average reduction in Moniezia eggs of 55% after treatment with injectable Praziquantel. Based on the above data results, it is evident that the potential for utilizing injectable Praziquantel to address cestode infestations in other Southern African ruminants justifies further research. It is recommended that a larger amount of Praziquantel be made available in order for the authors to perform a trial bigger than the current pilot, and perhaps use African wild ruminant herds as test populations.
Acknowledgements
Vermaak and C. Boshoff thank the owners of farms Lekkersmaak and Selwane for allowing the farms and herd of goats to be used for this study.