Pain and Fatigue Perceptions of Patients with Systemic Autoimmune Myopathies before and during the COVID-19 Pandemic Period ()
1. Introduction
The World Health Organization (WHO) declared SARS-CoV-2 disease (COVID-19) a pandemic in March 2020 [1]. The cases increased exponentially across cities, countries, and continents. COVID-19 resulted in aggressive policies such as nationwide lockdown or physical distancing policies to reduce spreading of the virus. [2] [3] In this scenario, remote work was adopted and people circulation restrictions aimed at essential and basic care (e.g., food and medical treatment) resulted in significant impairment in the general population’s mental and physical well-being [4] [5] [6].
In Brazil, during the first wave characterized by the March 2020-December 2020 period, physical distancing measures were adopted to reduce the spread of COVID-19. Nevertheless, due to a combination of several factors, and in part characterized by no adoption of nationwide lockdown policies, poor screening for new cases and contradictory policies in managing the pandemic, all lead to a decrease in adhering to social distancing. This resulted in an increase of the pandemic’s severity during the second wave [7] [8] [9].
Although necessary, restrictive measures assume a double-edged role, recent evidence shows that during the period of physical isolation there were significant increases in levels of anxiety, depression, and stress in the general population [10] [11]. Given this context, as priority groups (individuals susceptible to major complications due to a COVID-19 infection), patients with idiopathic inflammatory myopathies or systemic autoimmune myopathies (SAMs) comprise a group of rare and heterogeneous autoimmune rheumatic diseases [12] associated to impaired physical function and decreased overall quality of life which potentially be more effective by negative effects related the COVID-19 pandemic.
A single study with systemic autoimmune rheumatic diseases observed that 73% of the patients’ mental health worsened during the isolation period [13]. In this context, Meyer et al. [11] showed that the general population reported difficulties in handling emotions, as well as aspects related to self-care (e.g., physical activity versus sedentary behavior) [11].
Physical activity has considerable advantages as a self-care strategy to prevent several chronic diseases. Nonetheless, numerous evidence shows that beyond prevention, physical activity levels have been correlated with the clinical parameters of patients with autoimmune rheumatic diseases, as well as in SAMs [13] [14] [15] [16] [17]. London-Cardinal et al. [13] observed that the level of physical activity in patients with SAMs is associated with clinical status and muscle strength. In the same way, increasing the level of physical activity among these patients has been associated with clinical improvements in aspects related to mental health [13].
In this context, recent evidence has reinforced the need to have indicators related to mental health parameters [13] [14] [15] [16] [17]. Brady et al. [14] showed that physical activity level is associated with indicators of mental health and physical fatigue in patients with rheumatoid arthritis during the COVID-19 pandemic. In contrast, with regard to SAMs, due to the multifactorial component related to diseases, studies conducted through the Outcomes Measures in Rheumatology (OMERACT) group have suggested that patients’ pain and fatigue perceptions may be relevant indicators of disease’s impact and these patients’ quality of life [18] [19].
However, to the best of our knowledge, no studies have evaluated the relationship between pain and fatigue perceptions with disease status and whether sanitary measures associated with the COVID-19 pandemic interfere with these perceptions. Based on this, the study aimed to assess cross-sectionally the relationship between pain and fatigue perceptions with the disease parameters and prospectively evaluate the changes of these parameters during the COVID-19 pandemic.
2. Patients and Methods
Study design
This single-center study was compounded by two observational designs:
1) A cross-sectional analysis: Patients with SAMs were assessed between January 2020 and March 2020 and, therefore, before the Brazilian social distancing policies. In the same period, healthy individuals were also assessed.
2) A prospective analysis: Part of the patients with SAMs was re-evaluated between December 2020 and March 2021, during the return to daily routine medical care due to decrease in COVID-19 cases.
The study was approved by the local ethics committee (Hospital das Clínicas HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil - CAAE number 16768219.7.1001.0068) and all patients signed the informed consent form. In addition, the patient dta were performed in accordance with the Declaration of Helsinki.
Patients and procedures
Approximately 200 patients with SAMs are currently following-up at our hospital’s outgoing clinic. All patients with dermatomyositis (DM) and immune-mediated necrotizing myopathies (IMNM) fulfilled the European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) 2017 classification criteria for the idiopathic inflammatory myopathies, [20] whereas the patients with anti-synthetase syndrome (ASSD) fulfilled the criteria used by Behrens Pinto et al. [21].
Patients who did not understand the evaluations applied in the study and patients with extremely impaired functionality were excluded. Similarly, patients with myositis overlapping syndromes, cancer associated-myositis, and other myopathies were excluded from the present study (Figure 1).
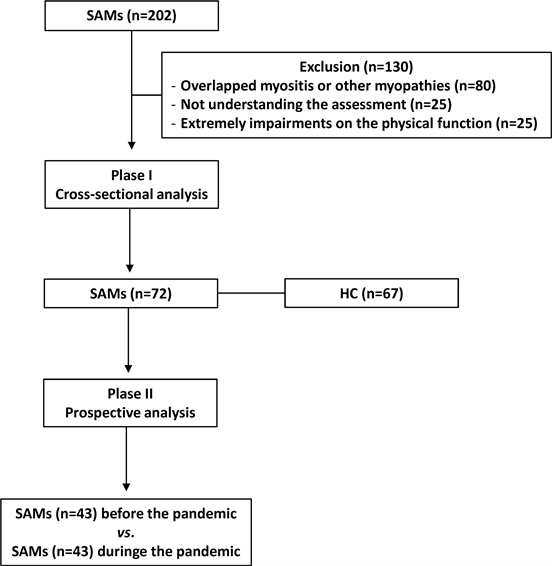
Phase I: Before the Brazilian pandemic (January 2020-March 2020) Phase ll: During the Brazilian pandemic (December 2020-March 2021). aPatients vs. healthy control group; bPatients before the pandemic vs. same patients during the pandemic. SAMs: systemic autoimmune myopathies; HC: healthy control.
Figure 1. Flowchart of the present study.
Patients engaged in a face-to-face interview with an experienced healthcare professional during routine outpatient consultations.
As a control group, healthcare professionals matched by age and gender were recruited and invited to fill in the questionnaires through an online form. Individuals with cardiovascular comorbidities or the presence of systemic autoimmune diseases were excluded according to the flow-chart (Figure 1).
Assessments. The following data were collected from the patients with SAMs:
· Demographic: current age, gender, education level, weight, height and body mass index (BMI).
· Laboratory data: serum levels of creatine phosphokinase (CPK) (reference values: 32 - 167 U/L). The identification of myositis-specific autoantibodies (anti-Jo-1, -Mi-2, -PL-7, -PL-12, -OJ, -EJ, and anti-SRP) and myositis-associated autoantibodies (anti-PM/Scl, -Ku, and anti-Ro-52) was performed using the commercial kit (Myositis Profile 3, Euroimmun, German) and the evaluation was based on the method conducted in a previous study [22].
· Disease status: disease duration and clinical assessment of SAMs were collected using scores proposed by the International Myositis Assessment & Clinical Studies Group (IMACS): Manual Muscle Testing (MMT-8), Myositis Disease Activity Assessment Visual Analogue Scales (MYOACT), global assessment of the disease through the Visual Analogue Scale (physician and patients), Health Assessment Questionnaire (HAQ), and muscle enzyme serum levels [23] [24] [25].
· Pharmacological treatment: including current treatment (glucocorticoids and immunosuppressive drugs) and associated medications.
· Comorbidities: systemic arterial hypertension, dyslipidemia, diabetes mellitus, depression, anxiety, and fibromyalgia. We considered hypertensive patients with systolic blood pressure ≥ 140 mmHg and diastolic blood pressure ≥ 90 mmHg or using antihypertensive drugs [26]. The diagnosis for dyslipidemia was total cholesterol > 200 mg/dL, LDL cholesterol > 130 mg/dL, triglyceride > 150 mg/dL, HDL < 50 mg/dL (women) or HDL < 40 mg/dL (men) or in use of drugs for the management of dyslipidemia [27]. For diabetes, changes in plasma glucose and use of pharmacological therapy were considered [28]; Fibromyalgia according to the classification criteria of the ACR 2010 [29]; Depression and anxiety through the criteria defined through the American Psychiatric Association [30].
· Perception of fatigue and pain: it was assessed using the VAS, ranging from 0.0 (absence of fatigue or pain) to 10.0 (presence of fatigue or severe pain) [31] [32].
· Fatigue severity: it was assessed using the fatigue severity scale, which ranges from 0.0 to 7.0, the higher the score the greater the fatigue [33].
· Level of physical activity and weekly metabolic equivalent (METs): it was measured using the International Physical Activity Questionnaire (IPAQ), which classifies it as a level of physical activity and the METs, classifying it: as a high level of physical activity for patients who comply with the recommendations of vigorous activity: (≥5 days/week and ≥30 minutes per session) or vigorous activities: (≥3 days/week and ≥20 minutes) and moderate activities or walking (≥5 days/week and ≥30 minutes per session). For classification of moderate, the patient must perform vigorous activities: (≥3 days/week and ≥20 minutes per session) or moderate activities or walks (≥5 days/week and ≥30 minutes per session), or the sum of any activities performed ≥ 5 days/week, and ≥150 minutes/week [34] [35].
All parameters previously presented were collected after one year from patients with SAMs. For the healthy individuals, demographic data, perceptions of fatigue, pain and physical activity level data were collected.
Statistical analysis. Data distribution was assessed using Shapiro-Wilk adherence test, the significance level adopted was α = 0.5. To analyze differences between clinical and demographic characteristics between groups, one-way ANOVA or Kruskal-Wallis tests were used. The categorical variables were used using the chi-square test or Fisher's exact test. The correlation between continuous variables was assessed using Pearson or Spearman tests. Weak correlation was considered with rho < 0.30 (no correlation); rho = 0.30 to 0.49 (weak), rho = 0.50 to 0.69 (moderate) and rho ≥ 0.70 (strong). P values ≤ 0.05 were considered statistically significant. Values of P ≤ 0.05 were considered statistically significant. The software used was SPSS version 25 (Chicago, IL, USA).
3. Results
Cross-sectional analysis
Out of 200 patients, 72 with SAMs and 67 healthy individuals as a control group were recruited at phase I. There were 130 patients excluded based on the inclusion criteria, according to the flowchart (Figure 1).
The demographic variables (age, gender, and BMI) were comparable between patients with SAMs and healthy individuals. The patient sample was composed of 36 (50.0%) with DM, 28 (38.9%) with ASSD, and 8 (11.1%) with IMNM (Table 1). The IMACS core set scores suggest that patients had stable or low disease activity. Additionally, one-third of patients were using prednisone with a low daily dose. The comorbidities and other current drugs are also shown in Table 1.
The pain perception the VAS assessed was significantly higher in patients with SAMs compared to healthy individuals. Two-thirds of the patients with SAMs had moderate or intense pain perceptions. Similar to pain perception, two-thirds of the patients had moderate or intense fatigue perception, but this was comparable to healthy individuals. The fatigue severity degree was also similar in both groups. There was no difference in low physical activity levels in patients with SAMs compared to healthy individuals.
Prospective analysis
After one year, 43 out of 72 patients with SAMs were assessed. Three patients died, and 26 were not assessed because of other reasons (e.g., missing the appointment due to pandemic; non-accessibility for contact by remote communications strategy). The sample consisted of 24 (55.0%) patients with DM, 13 (30.2%) with ASSD, and 6 (14.0%) with IMNM (Table 2). Therefore, the data from these 43 patients with SAMs were compared to the data from the same 43 patients from phase I (Figure 1).
For the BMI and disease status, treatment was comparable between both periods. The pain and fatigue perceptions, fatigue severity and physical activity levels were also similar in both periods.
During the follow-up, two patients and one patient received depression and fibromyalgia diagnosis, respectively (Table 2).
As an additional analysis, the correlations between pain perception with clinical status during the pandemic moderate correlated with patient’s VAS (rho = 0.61; P = 0.001) and there was a weak correlation with physical function (rho = 0.42; P = 0.004), and the fatigue severity scale (rho = 0.31; P = 0.042), and was
![]()
Table 1. General characteristics of patients with systemic autoimmune myopathies and healthy individuals
Data presented as mean ± standard deviation, median interquartile (25% - 75%) or frequency (%). ASSD: antisynthetase syndrome; BMI: body mass index; DM: dermatomyositis; FSS: Fatigue Severity Scale. HAQ: Health Assessment Questionnaire; IMNM: immune-mediated necrotizing myopathies; MMT-8: Manual Muscle Testing; VAS: Visual Analogue Scale. *Comparison between all patients and control group.
![]()
Table 2. General characteristics of 43 patients with systemic autoimmune myopathies at before and during following- up (COVID19 pandemic period).
Data presented as mean ± standard deviation, median interquartile (25% - 75%) or frequency (%). FSS: Fatigue Severity Scale. VAS: Visual Analogue Scale; HAQ: Health Assessment Questionnaire; MMT-8: Manual Muscle Testing; MYOACT: Myositis Disease Activity Assessment VAS. *Comparison between patients before and during COVID-19 pandemic period.
![]()
Table 3. The correlation between pain perception with anthropometric, clinical and laboratory data, comorbidities, and physical activity levels parameters of 43 patients with systemic autoimmune myopathies before and during following-up (COVID-19 pandemic period).
FSS: Fatigue Severity Scale. VAS: Visual Analogue Scale; HAQ: Health Assessment Questionnaire; MMT-8: Manual Muscle Testing-8.
inversely correlated with laboratory data (rho = −0.37; P = 0.014) (Table 3). The fatigue perception also correlated moderate with the patients’ VAS (rho = 0.64; P = 0.001), and weak with physical function (rho = 0.48; P = 0.001) and the fatigue severity scale (rho = 0.37; P = 0.014) (Table 4).
![]()
Table 4. The correlation between fatigue perception with anthropometric, clinical and laboratory data, comorbidities, and physical activity levels parameters of 43 patients with systemic autoimmune myopathies before and during following-up (COVID-19 pandemic period).
FSS: Fatigue Severity Scale. VAS: Visual Analogue Scale; HAQ: Health Assessment Questionnaire; MMT-8: Manual Muscle Testing-8.
4. Discussion
The present study shows 1) which patients with SAMs had increased pain perception when compared to the healthy individuals; 2) that during the COVID-19 pandemic period, no significant changes were observed on the anthropometric, clinical, laboratory, and pain and fatigue perceptions in patients with SAMs; and 3) that the pain and fatigue perception correlated positively with physical function and the disease visual analog scale.
As advantages of the present study, we included a representative sample of patients with SAMs. An aspect worthy of attention, the patients were undergoing a face-to-face interview and also using smartphone instant messaging, which allowed a more precise monitoring during all the study’s periods. Nonetheless, using validated questionnaires and directly assessing pain and fatigue leads to a better understanding of the impact of pain and fatigue on the overall quality of life. Another strong point is related to the detailed information of the patients’ comorbidities and general clinical status, avoiding the potential bias related to the pain and fatigue characteristics. Moreover, the patients showed a stable clinical status which correlates with the pain and fatigue perception and suggests further mechanisms beyond the disease-specific mechanisms.
Cross-sectional analysis
Our results are corroborated with previous evidence from the working group OMERACT [18] [19] [20] that constantly reinforce the role of the pain and fatigue in patients with SAMs. Nonetheless, a recent study based on the German database showed increased fatigue (57%) of the sample and impaired global health (60%) [36]. However, the main limitations from these studies comprises a lack of presentation of data related to clinical, pharmacological and comorbidities that have related parameters and lead to bias that is related to the fatigue and pain perceptions. Fatigue and perception are multidimensional symptoms closely associated with disease activity in rheumatic diseases such as Sjögren’s syndrome and systemic erythematous lupus [37] [38]. In this context, the fatigue and pain perception among patients with SAMs and their relationship between clinical parameters are unclear. Given this, our study showed that patients’ VAS with low-disease activity were associated with pain and fatigue perceptions, which suggests the role of underlying factors that influences the pain and fatigue perceptions. In the present study all patients had relatively preserved physical function and a stable disease from the clinical and laboratory point of view; concerning this context, the elevated fatigue and disease perceptions may be associated with a mechanism linked to maladaptive plasticity of the central nervous system. Interestingly, a substantial body of evidence has shown that a stereotypical pattern of responses denominated sick behaviors, which leads to functional changes in brain connectivity results in interoceptive alterations. The interoception detects bodily sensations coordinated through the immune and endocrine systems; changes in interoceptiveness can influence brain areas such as the mid-insulin, which is associated with the increase in fatigue and pain perception such as the pain chronicity [39] [40] [41] [42]. In the same sense, Lernia et al.’s study [42] showed that patients with musculoskeletal pain have a less interoceptive response. In fact, these results can corroborate our findings that patients with SAMs showed a significant difference related to the pain perception. In the rheumatic diseases’ context, Duschek et al.’ study [41] observed that patients with fibromyalgia had decreased interoceptive awareness. However, the subjective characteristics related to the disease, and pain and fatigue perceptions are not fully understood, thus reinforcing the need for future studies to better understand these features.
Prospective cohort analysis
The purpose of a prospective analysis to measure the changes related to the clinical parameter and physical activity during the COVID-19 pandemic. However, no significant changes were evidenced. Physical activity has been extensively associated with clinical improvement in rheumatic diseases and increases in physical activity levels are associated with decrease in the pain and fatigue [43]. Landon-Cardinal et al.’s recent study [43] demonstrates a correlation between physical activity levels and how changing physical activity levels have a role on these parameters. In this present study, no changes on the physical activity and clinical parameters were evidenced, these data can be attributed to factors of differences on the perceived physical activity with the objective physical activity previously observed [44] Nevertheless, the baseline and follow-up clinical parameters suggest low-disease activity and preserved physical function, while the pain and fatigue remain associated with clinical and physical function. Given this, Campbell et al.’s study [45] showed that strength and endurance do not necessarily reflect the fatigue in these diseases when assessed with fatigue specific instruments. These findings reinforce the necessity of managing pain and fatigue perceptions in these diseases.
The present study has some limitations. First, the healthy individuals were composed of health professionals who have increased fatigue perception according to previous studies [46] [47] and additionally were recruited during the pandemic period, which was characterized by having a negative impact on mental health in the general population [46] [47]. Second, the absence of objective measures regarding the pain and fatigue leads to uncertain results, thus the need for future studies to compare the difference between the levels of reported and measured objective physical activity and correlated to other parameters related to pain and fatigue in these patients.
In summary, this study showed significant differences concerning the pain perception between the healthy control group and the patients with SAMs. Moreover, during the pandemic, no changes were evidenced related to the clinical status, and pain and fatigue perception parameters. Nonetheless, the notable correlation between the pain and fatigue perception with the disease status was evidenced. The present study’s results reinforce the relevance of the subjective perception related to the presence of fatigue and pain; therefore, future studies are needed to understand the mechanisms associated with the disease perception, pain and fatigue, and the tools used for pharmacological and non-pharmacological therapeutic targeting to improve these parameters.
5. Conclusion
The study showed that patients with SAMs have significantly increased pain perceptions compared to healthy individuals. During the COVID-19 pandemic period, the pain and fatigue perceptions remained unchanged in patients with SAMs but they correlated to several disease status parameters.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP #2019/12155-5 to R.G.M., #2019/11367-9 to I.B.P.B., # 2020/10691-4 to A.M.S.; #2019/11776-6 to S.K.S.; Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq #303379/2018-9 to S.K.S.; Faculdade de Medicina da USP to S.K.S.